Sarepta: The AdCom Documents Enter The Discussion

Image Source: Pixabay
The briefing documents for Sarepta’s (SRPT) upcoming AdCom for its gene therapy to treat DMD were released today. There will be a lot written and discussed with even more during the AdCom but I do not want to add to that noise. I still believe that the real value-generating event for Sarepta will be the phase III data and I think the documents actually show some interesting data that can help us understand the potential success or failure of that trial.
What I think is absolutely critical is looking at whether or not the biomarker (changes in the level of microdystrophin) correlates with functional outcomes. The stronger that correlation, the better the odds of success in a larger trial. There seems less doubt as to whether the treatment impacts the levels of microdystrophin (it seems to clearly do that) but the question is whether that will lead to positive functional improvement for patients.
This is the correlation between the changes in microdystrophin and functional improvements (all of these figures come from the briefing documents and FDA analysis of the data).
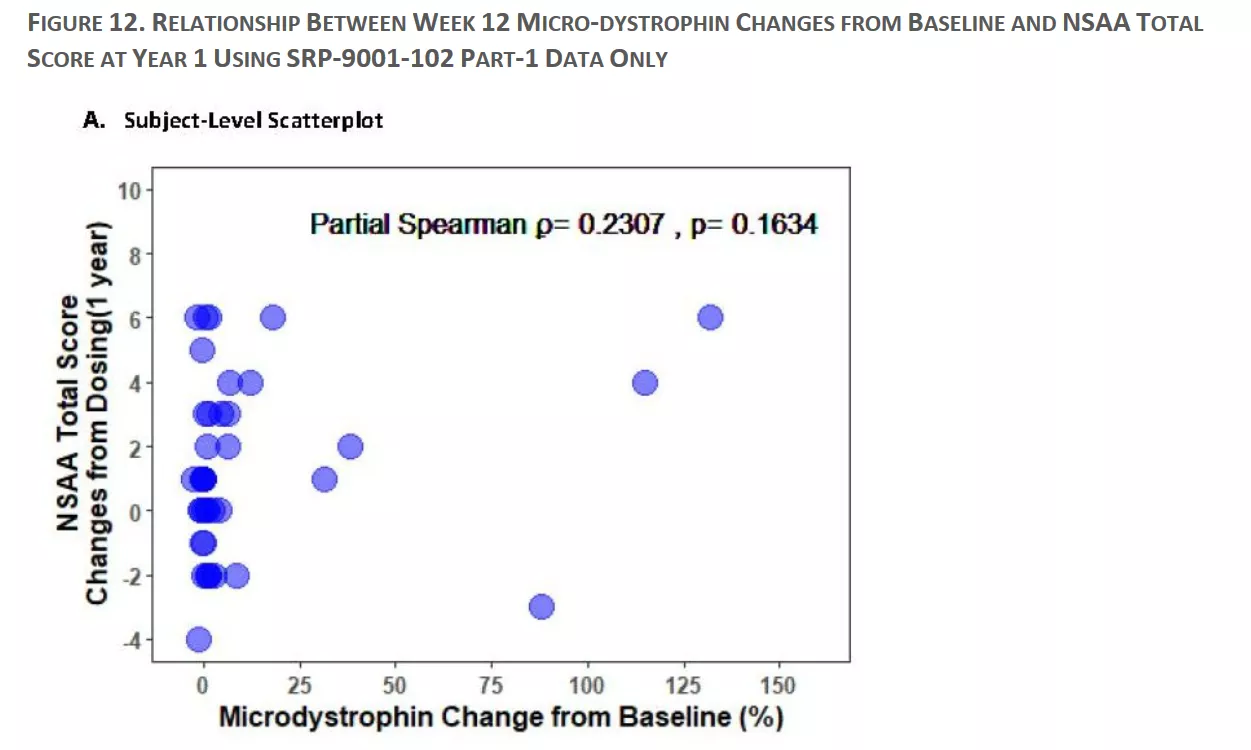
What we would want to see is a clear pattern, where higher changes in microdystrophin have greater improvements in NSAA total score. If we simply use the eyeball, there does not seem to be a clear relationship. The partial Spearman confirms this (a value of 0 means no correlation whereas a value of 1 would be a complete positive correlation) with a modest 0.23 and a p-value that is not statistically significant. In other words, the relationship looks weak, and any trend is not even statistically significant.
There are, however, some additional analyses that provide some context. This is a similar analysis but looks at patients from two different age groups (4-5 years old and 6 or older).

This is easier to eyeball as all you need to do is make a line from the placebo dot to the treatment dot for each age group. What you want to see is a positively sloped line (which would be more microdystrophin leads to a greater functional improvement). What is striking is that there is basically no effect in the older group (it appears slightly negative) but a much stronger effect in the younger group. You find a similar effect when looking at the pooled data.
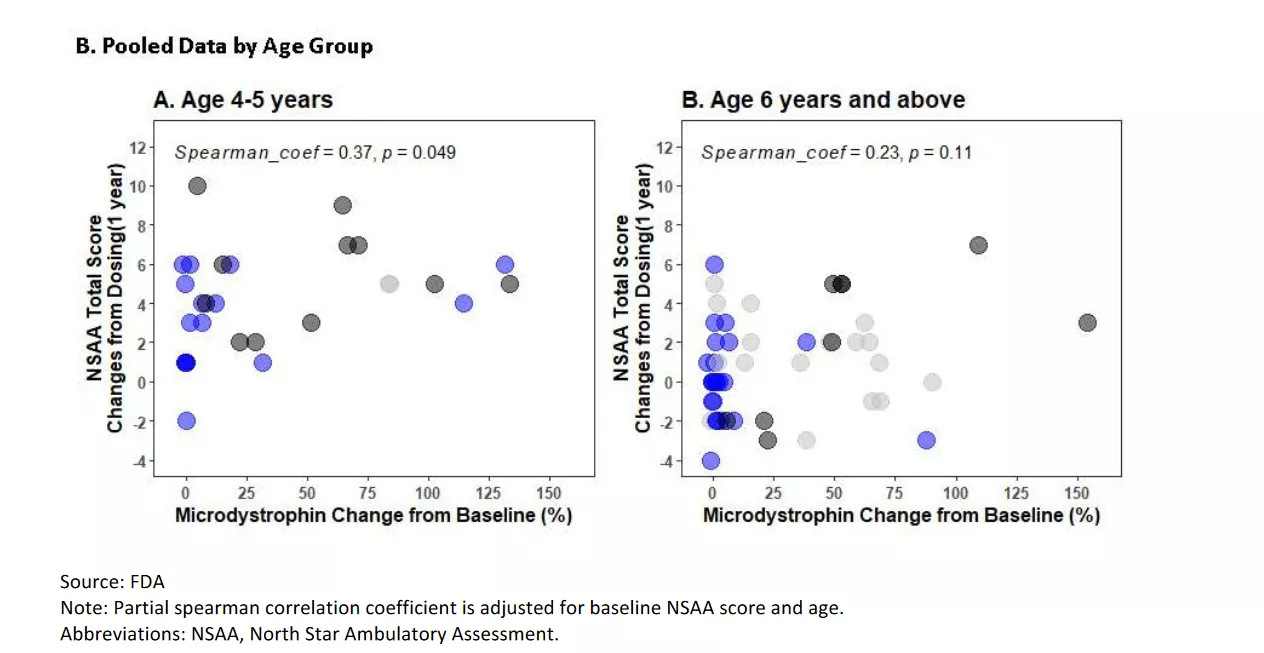
Note not only the difference in the Spearman correlation coefficient, where it is higher in the younger group, but it is also statistically significant in the younger group. This makes sense in that intervening when the disease is less well established might lead to more benefit but clearly, it looks like the younger group is key.
This leads me to the phase III data. I would want to know the distribution of ages in the trial. The more that trial has a younger patient population, the greater the chance that it shows a clear positive benefit. I do think the trial is large enough that even if the whole population does not show a benefit, they could do a sub-set analysis but in terms of the topline results, I think these data show that the younger the population in that trial the greater the chance of a positive top-line result.
I would probably be remiss if I at least did not mention the potential AdCom results and I suspect the following analysis could be quite influential. This is basically the aggregate analysis by the FDA of the relationship between changes in microdystrophin and functional benefit.
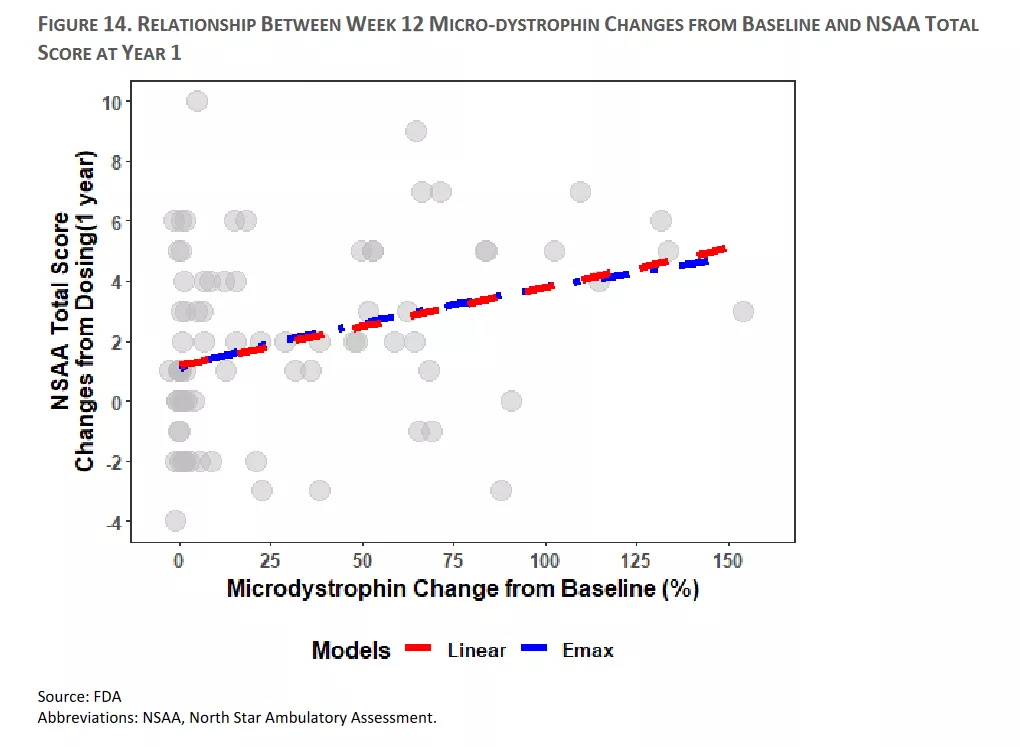
This figure basically shows that increases in the microdystrophin level at week 12 are correlated with functional improvements at year 1. One can argue about the effect size but the analysis indicate that every 10% improvement in microdystrophin leads to a 0.26 benefit at year 1. In addition, this effect is statistically significant with a p-value of 0.0038.
Again, I do not want to wade into the AdCom vote predictions or the FDA politics around this issue but if there is a positive vote, then I think that figure likely becomes important (but I am not making a call one way or another). My bigger point is that the FDA analysis if the data implies that the age distribution of the patients in the phase III trial likely impacts the odds of a positive top-line result, which I think is important to understand.
More By This Author:
Another Sucess In Alzheimer’s Disease
Biogen On The Hunt
Coya Therapeutics: An Attractive Valuation And First Set Of Data
Disclosure: No position in stocks mentioned.
Investment and investment decisions should be based on your own personal needs and with the consultation of a trained financial advisor. Any ...
more


