Akari Therapeutics' Nomacopan Is A Potential Blockbuster

Image Source: Unsplash
Akari Therapeutics (Nasdaq: AKTX) is a clinical stage biotechnology company developing advanced therapies for autoimmune and inflammatory diseases. It’s lead drug candidate, investigational nomacopan, a bispecific recombinant inhibitor of complement C5 and leukotriene B4 (LTB4), has potentially wide applicability and blockbuster potential.
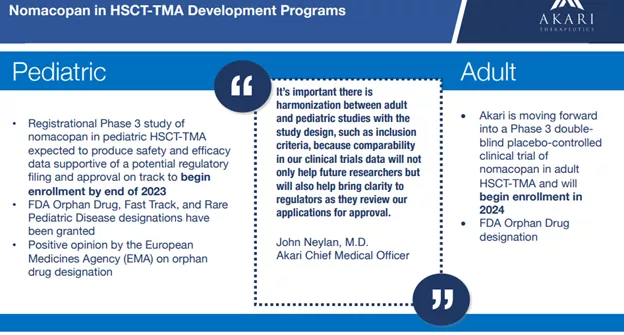
Nomacopan is in Phase 3 development for pediatric hematopoietic stem cell transplant-related thrombotic microangiopathy (HSCT-TMA) and is entering Phase 3 development in adult HSCT-TMA, so a first approval is potentially on the horizon.
While the company will require additional capital to fund its clinical program, its cash needs are not huge, and its market cap is a modest $29M (fully diluted).
Akari has a near-term pipeline of two diseases for its lead drug candidate, nomacopan.
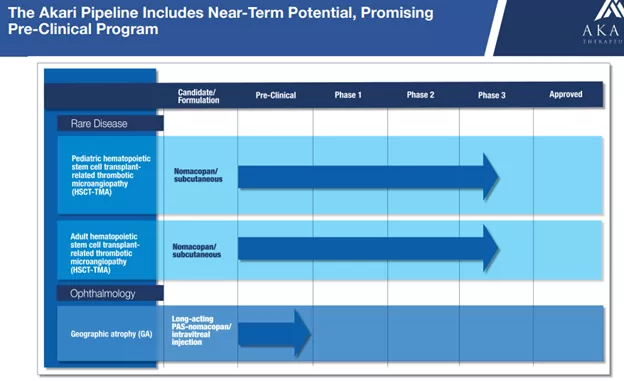
Nomacopan
Nomacopan is a bispecific recombinant inhibitor of complement C5 activation and leukotriene B4 (LTB4) activity.
Nomacopan works in a dual way blocking both C5 and LTB4. It acts on complement component C5, preventing the release of C5a and formation of C5b–9 (also known as the membrane attack complex, or MAC), and also inhibits leukotriene B4, or LTB4, activity.

No serious side effects have occurred with nomacopan in clinical trials. Nomacopan has been studied in various routes of administration including subcutaneous, intravitreal, intravenous, and even inhalation. It has broad potential and has been studied in diseases including:
- HSCT-TMA or hematopoietic stem cell transplant-related thrombotic microangiopathy
- Geographic atrophy (GA)
- Paroxysmal nocturnal hemoglobinuria (PNH)
- Atypical hemolytic uremic syndrome (aHUS)
- Bullous pemphigoid (BP)
- Uveitis
- Atopic keratoconjunctivitis (AKC)
- COVID-19 pneumonia
- Traumatic brain injury and hemorrhagic shock
- Lung diseases
Nomacopan has received regulatory designations in the U.S. and Europe, including Orphan Drug, Fast Track and Rare Pediatric Disease designations and orphan drug designation from the European Commission.
However, given limited funds management decided in August 2022 to concentrate efforts on HSCT-TMA and GA.
HSCT-TMA
HSCT-TMA is a rare and severe condition. It is a form of TMA (thrombotic microangiopathy) that is triggered by HSCT (hematopoietic stem cell transplantation).
HSCT-TMA can be a severe complication of HSCT with a case-fatality varying between 50% and 90%.

There are no specific diagnostic tests for HSCT-TMA, however routine blood tests and evaluation of blood cells under a microscope can help make a diagnosis. There have been efforts to improve this (company PR):
Consensus guidelines were published in early 2023 by an international panel of experts that harmonize diagnostic criteria and support earlier screening and diagnosis in the care of patients. The design of Akari’s HSCT-TMA Phase 3 clinical trials has been significantly informed by these consensus criteria for earlier diagnosis of high-risk (severe) patients.
There are no approved treatments for adult or pediatric HSCT-TMA patients in the US or Europe.

Akari has been granted Orphan Drug, Fast Track and Rare Pediatric Disease designations from the FDA for nomacopan for the treatment of pediatric HSCT-TMA. The Rare Pediatric Disease Designation provides the company with a Priority Review Voucher (PRV) at approval, which it can use for an upcoming review (or sell to a third party).
The company’s earlier trials (1a and 1b) showed safety and tolerance and determined the range of doses, but were too small to produce meaningful results in terms of efficacy.
The company conducted a Phase 2 trial with nomacopan for bullous pemphigoid (BP) produced positive results:
In this nonrandomized controlled trial of 9 patients with bullous pemphigoid, nomacopan was well tolerated in all patients for 42 days. In 7 of 9 patients, treatment with nomacopan was associated with a significant decrease in disease activity, including near complete remission in 3 patients.
The company is finally embarking on Part B of its Phase 3 clinical trial.
The trial consists of two parts:
- Part A is a dose confirmation study, followed by
- Part B which is the pivotal responder-based safety and efficacy study
Two of three age groups for Part A have already been enrolled with the third age group (the youngest, 0.5-2 years) still enrolling and patients will be enrolling for Part B of the trial by year-end.
What we know is that nomacopan is safe and well tolerated and the company has received substantial backing from regulators (Orphan Drug, Fast Track, and Rare Pediatric Disease designations).
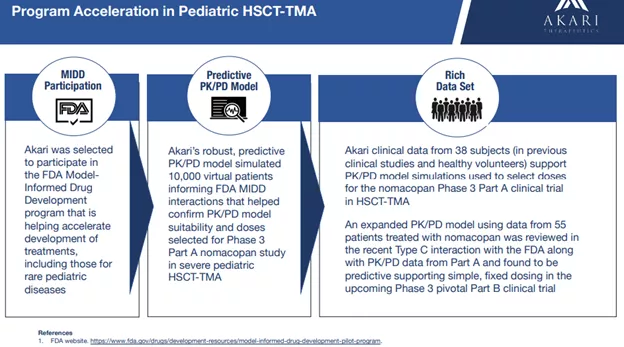
The company has also built a predictive model based on prior trials resulting in a simple fixed dose in the upcoming Phase 3 trial:
There is also the case of a successful 6 year old patient who completed treatment in Part A of the Phase 3 trial (company PR):
A 6-year-old male HSCT-TMA patient at Royal Manchester Children’s Hospital, Manchester University NHS Foundation Trust in Manchester, United Kingdom was enrolled in the nomacopan Phase 3 Part A clinical trial, treated with nomacopan and discharged home from the hospital.
He remains well and in remission.

The case study is encouraging, but of course not remotely a substitute for a pivotal trial like the one coming up.
There will also be a Phase 3 trial for adult patients suffering from HSCT-TMA that will begin enrollment next year.
Geographic Atrophy (GA)

The first treatment for GA, Syfovre (monthly or bi-monthly injections) was approved by the FDA early in 2023, followed by a second treatment, Izervay (monthly injections), in August this year.
Current standard of care has some disadvantages, which is where Akari’s long-acting PAS-nomacopan could provide an additional option:

That is, long-acting PAS-nomacopan could reduce the number of injections needed to 4x a year. And, due to its dual action, it could also reduce the risk of a side effect of the other approved treatments, including patients developing CNV (sight-threatening choroidal neovascularization). From the 20-F:
Dual-action PAS-nomacopan may offer the well-understood benefits of complement C5 inhibition in slowing the progression of GA lesions, while LTB4 inhibition also has the potential to help prevent VEGF-A over-expression, a key driver of CNV. These positive results support the potential of long-acting PAS-nomacopan to advance toward IND/IMPD for clinical trials in GA / advanced dry AMD. The program is on -track to submit an IND to the FDA in the first half of 2024.
The company expects PAS-nomacopan pre-clinical research will be completed in short order and development will advance into clinical trials in 2024, from the earnings PR:
Akari also selected Wacker Biotech GmbH as the manufacturing partner to support production of PAS-nomacopan for use in clinical trials. The company remains on track for an Investigational New Drug (IND) submission to the FDA in the first half of 2024 and the start of clinical trials in the second half of 2024 (subject to funding).
This will take time, there aren’t yet any clinical trials planned, but it’s another opportunity for nomacopan, and it’s not the only one.
Other conditions

The company embarked on a Phase 1/2 trial with nomacopan for patients with inflammatory mediated eye disorder atopic keratoconjunctivitis, or AKC in 2018. The Part A (involving three patients) showed no serious adverse events, and the eye drops were well tolerated in all three patients.
The Part B consisted of a randomized, double-masked placebo-controlled comparison in 16 patients was halted due to the pandemic but for 10 of the 12 patients data was available, although 6 of these were on a placebo, from the 20-F:
Aggregating data from the eight AKC patients from Part A and Part B shows no ocular treatment emergent serious adverse events during the eight-week treatment period… Although the four nomacopan treated AKC patients achieved a higher improved mean efficacy score than the four placebo AKC patients, the patient numbers are too small to show statistical significance on efficacy measures between the two treatment groups.
The company was going to initiate Pivotal Phase 3 study for treatment of Bullous Pemphigoid with nomacopan after the success with the Phase 2 trial but instead focused the pipeline on HSCT-TMA and GA.
In April 2023, the European Patent Office granted a patent for nomacopan in the treatment of autoimmune blistering diseases (including bullous pemphigoid).
Preclinical data comparing the therapeutic efficacy of nomacopan, long acting PAS-nomacopan, and a monoclonal anti-VEGF antibody administered in eye drops showed (20-F):
PAS-nomacopan was found to reduce intraocular VEGF levels by as much as the anti-VEGF antibody with 74% (p=0.04) and 68% (p=0.05) reductions respectively, compared to saline control. Furthermore, based on a clinical score measurement, inflammation increased in both the control and anti-VEGF groups by 49% and 33%, respectively, PAS-nomacopan treatment showed a 9% reduction in inflammation assessed by retinal fundoscopy (p=0.02).
This is an encouraging result showing the potential of nomacopan for the treatment of back of the eye diseases.
In 2020 the company published results from a two-year research collaboration with the UCL Institute of Ophthalmology for the use of long-acting PAS-nomacopan which showed that it mitigated both the severity and progress of retinal damage in two models of autoimmune uveitis.
Further, in 2022, the company presented positive results from two pre-clinical studies with PAS-nomacopan in eye diseases (one of these studies involved GA discussed above).
There were also encouraging results from a trial with hospitalized patients suffering from COVID-19 pneumonia (see here).
Financials
The company doesn’t generate revenue yet so it’s mostly an exercise in managing expenses and cash here, with the following expenses (for H1):
- R&D $3.3M (versus $5M in H1/22)
- G&A $6M (versus $6.1M in H1/22)
There was a $9.2M loss from operations and the net loss was only $3M due to a $6.1M change in fair value of warrant liability.
The company had $7.2M in cash at the end of Q2 and received a gross $2M from a private placement (and a $2.5M tax credit in the UK).
There were 5.06M shares outstanding at the end of H1, to which we have to add the roughly 600K additional shares from the recent private placement and the warrants:
(Click on image to enlarge)
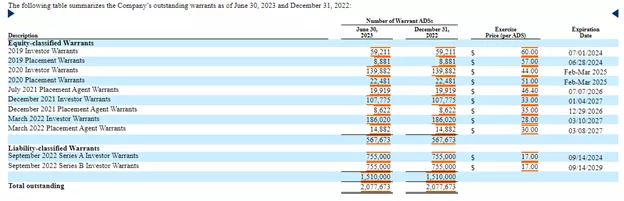
There are also options:
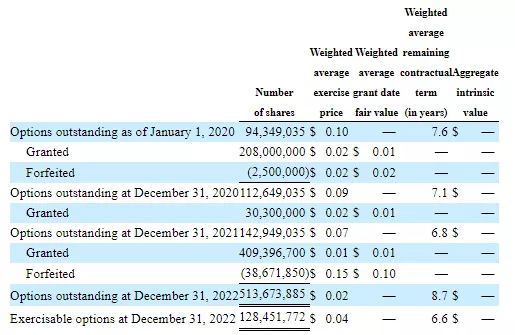
NOTE: The below is the most up to date information so please consider replacing charts above with this chart from the June financials and draw from this which gets to the $29M market cap.
680,112,400 options = 340,056 ADSs
4,155,347,500 warrants = 2,077,673 ADSs (same as above revised print screen on warrants)
418,580,700 RSUs = 209,290 ADSs
BASED ON ABOVE, A FULLY DILUTED NUMBER AS OF 6/30 (AND ADDING IN SEPT PIPE) WOULD BE 5.06M + 0.34M + 2.08M + 0.21M + 0.6M = 8.31M x 3.5 = $29.0M]
(Click on image to enlarge)
The company will undoubtedly need additional financing so the share count is likely to increase, something to keep in mind.
Conclusion
- Nomacopan, a complement inhibitor with dual working, is a potential blockbuster with widespread use and already an established safety profile.
- Its first target market, HSCT-TMA which has a very high mortality rate and for which there are no known treatments, is already advanced as the company is embarking on a pivotal trial shortly. It benefits from a number of regulatory designations like Fast Track, Rare Pediatric Disease Designation, and Orphan Drug Designation.
- While a positive outcome can’t be guaranteed, things look promising.
- Although the company will need additional financing, its cash needs aren’t huge and it has a moderate market capitalization.
More By This Author:
SaverOne Gains Rapid Traction With Its DDPS Solution
Investing In Luxury: Adamas One Unlocks The Potential For Lab-Grown Diamonds
Evogene Offers An Excellent Risk/Reward Ratio
Disclosure: This article is part of a new “UnderCovered” series of exclusive articles featuring companies with limited coverage. Authors are compensated by TalkMarkets for their ...
more



Very impressive.
Very good read, thanks.
You have some very good articles. The amount of research you do is very impressive.
Thanks Susan, appreciated.
So what is the total addressable market for this drug, when factoring in the other potential treatments?
Well, that really depends on pricing and as far as I'm aware, no other treatment is available for HSCT-TMA, they got orphan drug designation
Impressive!