Wednesday, October 14, 2020 1:56 AM EST
|
The 'experience' of this market - today centered on Apple (AAPL), LightPath (LPTH) and in late day developments, Sorrento (SRNE), at least from our perspective, and two of them had news. Along the way, the NIH Paused the Lilly (LLY) antibody trial, and of course media pondered if that will cause consumer resistance to antibody as well as (already encountering issues) vaccine treatments or vaccinations.
I have emphasized a preference as far as 'urgency' for antibody therapeutics, at the same time noted that Sorrento's candidates (of which another Clinical Trial was posted today) are (as far as I know) the lowest dose of all of them. It makes a real difference in potential side effects or toxicity. The President got 8 grams (I thought 4, but it was 2 doses, so 8), whereas Sorrento's is a fraction of that, hence 'scale' as well (quantity per amount of product) vastly better.
|
|
|
We repeatedly noted, and believe the public and media, seriously need to get a handle on the significance of 'dose loading' as they compare the treatments.
With 'odds' of safety/toxicity LOWER with Sorrento's antibody due to smaller comparative sizes of infusion, and that's the case in 'all' their candidates, they really should emphasize that in vying for Government funding.
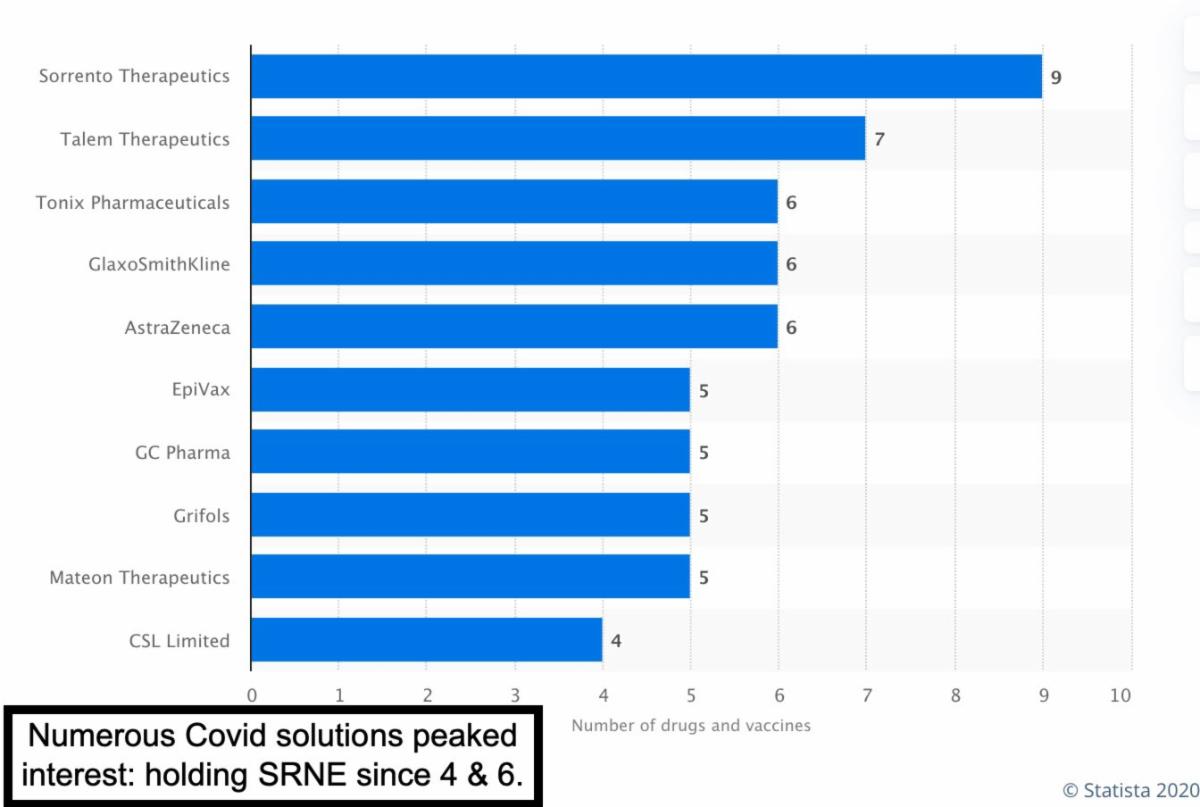
Instead on just cheering-on that this opens even more possibilities of market participation domination to Sorrento, I'd like to say it would be welcomed in the other companies worked also. Meanwhile Sorrento has filed for Phase 1 and 2 for a Generation 3 antibody, which I believe was only hinted at earlier.
Their presentation was a good update and put things in-order, even though it sold-off the stock, too many expecting some approval or funding announced at an R&D presentation I guess. That's realistically not how it goes, while at the same time it looks like they really do have promising prospects.
|
|
|
|
|
Executive summary:
- The Lilly antibody testing halt reminds everyone how difficult developing vaccines or therapeutic drug can be, perhaps enthusiasm Pres. Trump shared about his Regeneron (REGN) experience got people too optimistic.
- Personally I think not so, because dose levels matter as I've pointed out, minimal doses decreases side-effect risk, and that's been a focus Dr. Ji at Sorrento has tried to consistently point-out, even if analysts don't entirely appreciate the significance of (say) a 100 mg dose vs. a 4 gram dose like the President received.
- Sorrento announced a new Clinical Trial 'and' it's Phase 1 and Phase 2 for their 'AMG' (high performance?) advanced version of their antibody.
- I must note Apple, which had expected profit-taking (but mildly so), has a good lineup of 5G iPhones, they did not differentiate so it seems phones support 'all' bands with regard to carriers (they did emphasize Verizon as it introduces low-ban nationwide, probably the same AT&T (T) already had, at the same time Verizon (VZ) did surprise with at least 'parts' of 19 cities that will get the super-fast (higher band to keep this simple) cities.
- Best features on the iPhone 12 to me: the ability to automatically drop 5G to LTE to conserve battery where a customer isn't using fast capabilities, a series of camera improvements (of interest mostly to the Pro category, like image stabilization), and the new 'MagSafe' for wireless charging.
- iPhone 12 can be pre-ordered this week, Pro models not until Nov. 6th.
- The 'mini' model was actually most appealing, but I know I'm spoiled now and using my 'Max' for so much, that my eyes wouldn't be pleased with it.
- A small model of HomePod was revealed, but personally with a full-size HomePod that sounds fine, I think I'd be disappointed if the 'mini' model sounded as cloudy as the Amazon (AMZN) Echo Dot or something like that.
- Sorrento remained the day's core event given the R&D Presentation after the market's close (visiting LightPath was a chance to familiarize myself with the new team, and impressed at some greater manufacturing as well as engineering capabilities than I recall since my last visit awhile ago).
|
|
|

|
|
Meanwhile . . . anything can happen in technology or biotech, as years of elation, disappointment, and even comebacks surely testify too. That's as true for an Intel as it is for a company like LightPath (more as time allows and as developments merit), or as it is for even Sorrento.
|
|
|
The week's experience with pharmas having safety trials halted attests to how iffy the field can be, and this is a terrible time for disappointment that impacts all humanity. Sorrento focused on the very best medicine, and not fastest trial to get to market. Even in chats with LightPath (very deliberate and I believe creative in efforts expanding on what is already steady growth) we happened to discuss vaccines and the belief that initial offerings by major pharmas are more of an effort to attack the virus, not prevent the disease. I'm mentioning this given the engineering background of CEO Rubin, and his familiarity with scientists here in the US and in China, where he built a serious business from almost nothing in several years.
|
|

|
(At LightPath today: with Sam Rubin, CEO with 20 years in optics.)
|
|
|
Back to coronavirus. today The U.S. Patent Office (this is new) awarded two Patents to Sorrento Therapeutics. The Patents are: 1) Antibody therapeutics that bind CD38, and 2) Disulfide bridging conjugates. This matters even if the market is overly focused on day-to-day trading in this controversial biotech as it gets much closer to cash-flow positive new COVID-related solutions.
I would describe Sorrento's R&D Presentation (just ended, will evaluate more over time of course), as a solid rundown of ongoing and developed 'solutions', not just in COVID-19, although that's the market's focus at the moment. Also I'd think Dr. Ji wanted to reestablish faith with shareholders after a strange period given (what I too called hype or over-enthusiasm) before their solutions were more mature. However, that allowed our attractive entry level.
|
|
|
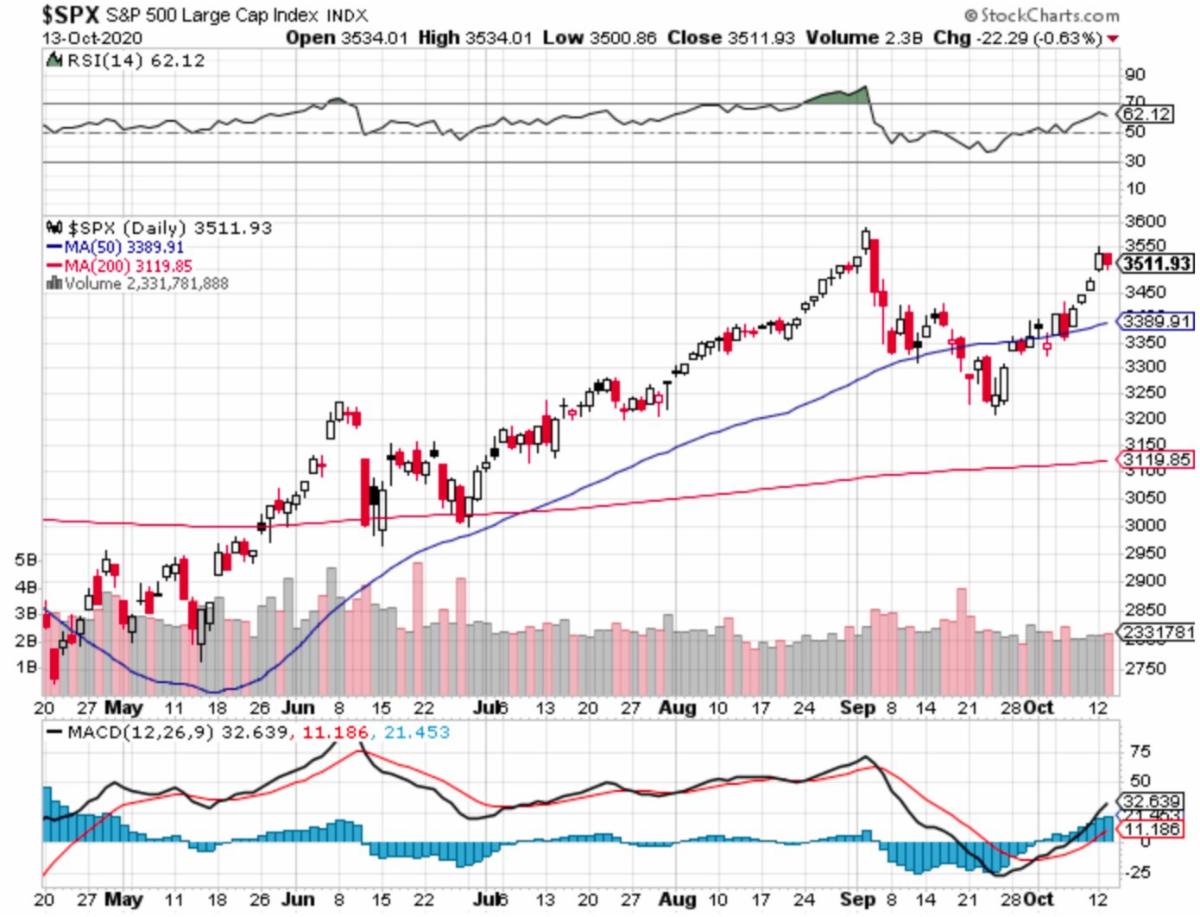
In-sum: Tuesday's S&P (SPX) behavior was about an anticipated, choppy with a bit of late comeback, with nothing earthshaking as far as the market itself.
|
|
How did you like this article? Let us know so we can better customize your reading experience.




interesting.