VBI Vaccines – The Leading Product In The Race For A $3 Billion Market
TM Editors' Note: This article discusses a penny stock or microcap. Such stocks are easily manipulated; do your own careful due diligence.
If you are starting to think that as equity investors we have had it too easy for too long you aren’t the only one. From the devilish 666 low on the S&P 500 back in March 2009, this entire index of very large stocks has gone straight up.
Really good individual stock picks are supposed to have that kind of increase. The index of the 500 largest companies isn't.
Putting money into passively managed funds has never been more rewarding. Last year, investors pulled $207 billion out of actively managed funds and put a record $413 billion into passively managed funds.
We hate to say this because we ourselves are retail investors. But what the retail "herd" is running away from is usually where you should be looking to invest. And what the retail herd is running towards is what you would normally be best advised to avoid.
In this case, that would mean we should be getting interested in investments outside of the S&P 500 and getting away from the passive funds that mimic the performance of the index.
It is time to roll up our sleeves and start picking some stocks.
VBI Vaccines – A First In Class Product For A $3 Billion Market
VBI Vaccines (VBIV) is a biotechnology company that is involved in the development of vaccines to prevent the spread of infectious diseases as well as therapeutic vaccines to treat cancer.
There are three different opportunities that the company is exploring and we will break them down by their potential long term value. There is also another opportunity related to a health concern that has been very much in the news of late.
Opportunity #1 - CMV (Cytomegalovirus) Vaccine
VBI is developing a prophylactic vaccine to prevent cytomegalovirus (CMV) infection. You may not have heard of CMV, but that isn’t because it isn’t prevalent.
CMV is a common virus that infects one in every two people in many developed countries. Most CMV infections are “silent,” meaning most people who are infected with CMV exhibit no signs or symptoms.
Therefore CMV isn’t a problem for most of us. Where it becomes a problem is when a mother is infected during pregnancy and it results in serious consequences for the newborn. This is known as a congenital CMV infection.
Source: VBI Corporate Presentation
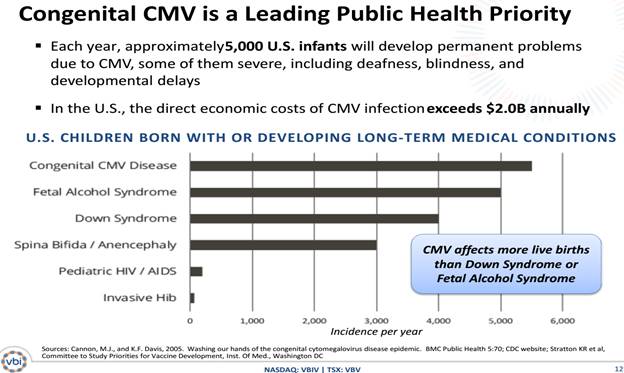
Each year, approximately 5,000 U.S. infants will develop permanent problems due to CMV, some of them severe, including deafness, blindness, and mental retardation.
CMV affects more live births than Down Syndrome or Fetal Alcohol Syndrome, making it a key public health priority and a strong candidate for recommended universal vaccination among certain high-risk populations.
There is no vaccine for prevention of CMV and VBI is likely a year to 18 months ahead of its nearest competitor vaccine which is being developed by Merck
Should VBI successfully develop a fully tested and approved vaccine there is likely to be widespread public health support for it.
The market for this product is estimated to be $1.5 billion in the United States (based on number of births and $100 per vaccination) with a similar sized opportunity in developed markets outside the United States.
That would give VBI the inside track on a $3 billion market.
Opportunity #2 – Commercially Approved Sci-B-Vac Hepatitis B Vaccine
Sci-B-Vac came to VBI as part of a recent merger with SciVac.
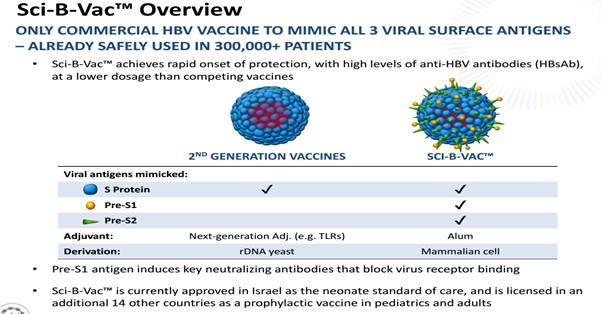
This merger combined VBI’s early stage opportunities with SciVac’s commercialized HBV vaccine called Sci-B-Vac. Sci-B-Vac is already approved for use in 15 different countries but not in the large markets of the United States and Europe.
A big part of the hold-up was that SciVac did not have the capital resources to invest in clinical and regulatory strategies to move it towards approval in these large markets.
Now as part of VBI management can move quickly to initiate an approval process in Europe and the US.
The target of this vaccine is Hepatitis B.
Hepatitis B is usually spread when blood, semen, or other body fluids from an HBV-infected person enter the body of someone who is not infected. HBV attacks the liver, causing both acute and chronic disease.
In its early stages, chronic hepatitis B infection is largely asymptomatic and many patients who may benefit from treatment go undiagnosed. Research has shown that treatment can have a major preventive effect on liver-related mortality and morbidity in persons with chronic hepatitis B infection. Efforts are needed to detect those infected who would benefit from treatment, so that the costly long-term side effects of HBV can be better managed.
According to the World Health Organization, globally, 240 million people are chronically infected with HBV and more than 780,000 people die every year due to complications related to HBV, including cirrhosis and liver cancer. In sub-Saharan Africa and East Asia, 5-10% of the adult population suffers from chronic hepatitis B infection. High rates of chronic infections are also found in central and eastern Europe, the Middle East, and the Indian subcontinent.
Sci-B-Vac is a licensed third-generation hepatitis B vaccine that has demonstrated safety and efficacy in over 300,000 patients.
Source: VBI Corporate Presentation
Opportunity #3 - Glioblastoma Immunotherapy
Glioblastoma is among the most common and aggressive malignant primary brain tumors in humans. In the U.S. alone, 12,000 new cases are diagnosed each year.1
The current standard of care for GBM is surgical resection, followed by radiation and chemotherapy. Even with aggressive treatment, GBM progresses rapidly and is exceptionally lethal, with median patient survival of less than sixteen months.2
VBI has applied its eVLP Platform in the development of a glioblastoma therapeutic vaccine candidate. With its novel approach, VBI intends to create a GBM immunotherapy that will stimulate the patient’s own immune system to identify and kill GBM cancer cells, with the goal of creating a commercially-viable therapy that is more effective and tolerable than current treatments.
Late Breaking News – Another Opportunity
This month VBI shared that it planned to develop a vaccine to combat the Zika virus using the company’s eVLP platform.
This vaccine will use the E glycoprotein found on the surface of the Zika virus and the NS1 glycoprotein secreted through Zika replication.
VBI’s management is currently looking at several different eVLP vaccine options and want to have an official vaccine candidate by October of this year. By the first quarter of 2017 VBI would look to enter clinical development in the first half of next year.
Who knows what this might be worth if anything but it is something to be aware of.
VBI’s Management and Board Of Directors - An Experienced And Credible Bunch

Source: VBI Corporate Presentation
Steven Gillis is Chairman of the Board of Directors. Gillis was a founder and director of Corixa Corporation and served as the company's CEO from its inception and as its Chairman from 1999 until its acquisition in 2005 by GlaxoSmithKline. Prior to Corixa, Steve was a founder and director of Immunex Corporation. From 1981 until his departure in 1994, he served as Immunex's Director of Research and Development, Chief Scientific Officer, and as interim CEO following Immunex’s majority purchase by American Cyanamid.
As Chairman he is representing Arch Venture Partners which is a Venture Capital firm focused on the biotechnology sector with nearly $2 billion in assets under management.
VBI’s Chief Executive Officer is Jeff Baxter who was a senior officer at GlaxoSmithKline with whom VBI has an important collaborative relationship.
Back in May of this year VBI merged with SciVac Therapeutics. That brought David Anderson (formerly SciVac’s CEO) into the VBI fold as Chief Scientific Officer.
That merger also brought in two new Directors from OPKO Health (OPK) which owned a significant portion of SciVac.
Five institutions control nearly 50% of VBI’s shares. Those institutions are Opko Health, CLS Therapeutics, Clarus Ventures, Arch Advisors and Perceptive Advisors.
VBI Vaccines – The Key Facts
Share Price - $3.50
Shares Outstanding – 36 million
Market Capitalization - $126 million
Net Cash - $15 million
Enterprise Value - $111 million
Risks - To Be Taken Seriously
This is a development stage company with no positive cash flows and very little revenue. While the potential here is intriguing given the company's positioning in a large unaddressed market, this kind of business is inherently risky. Please keep that in mind.
Disclosure: I have no position in this company.




VBI Vaccines being focused on several unmet biotech needs like CMV vaccine, GBM immunotherapy, and Zika virus vaccine, backed by strong institutional investors, has made this company worth looking for in biotech space.
The history has shown us that small-cap companies can grow exponentially once it gets its product approved in the huge market. According to the experts, VBIV could be one of those companies.
I plan to look into the VBI Vaccines, to better understand their future value and determine if this penny stock is a good investment.
Find any good picks in your research that you'd like to share with the rest of us?
I'd like to know about some good #vaccine stock pics as well.
Interesting to read about the projects that VBI is currently working on. It is a field with lots of opportunity so investors will no doubt be keeping their eye on future plans.
I agree, I think there is a lot of upside potential here.
Looks like VBI has a variety of projects on tap. I hope we will see more companies researching treatments for brain tumors and other aggressive cancers. I will be curious to follow the progress of these researchers on the different ideas mentioned above--I wonder which of these will prove to be the most promising in terms of research/development over the next few years.
This article is a good read because I've worked with a lot of special needs children over the years. No one can ever really predict CMV or down syndrome while the baby is developing in the mother, however for CMV it's apparent in the few short years after birth. The children do have a harder time learning in the classroom, and usually have some sort of autism. If the biotech world can help these children earlier in life, to rid this world of cancer, and other birth defects just imagine what this world could look like? It's definitely an exciting topic to follow for the years to come.
Great to see that there can be a potential vaccine for cancer. This should definitely change technology as we know it.