SciSparc Might Become The Next GW Pharmaceuticals

With the Jazz Pharmaceuticals buyout of GW Pharmaceuticals, biotech marijuana stocks are in the spotlight and one of particular interest is SciSparc Ltd. which is undertaking a study of a formulation of cannabinoid (CBD) and Palmitoylethanolamide (PEA) for treating symptoms of autism spectrum disorder and epilepsy and their preliminary research has demonstrated its efficacy as compared to the effect of CBD alone.
Might SciSparc become the next GW Pharmaceuticals?
About SciSparc
SciSparc Ltd., headquartered in Tel Aviv, Israel, is a specialty, clinical-stage pharmaceutical company focusing on the development of cannabinoid-based treatments.
SciSparc's First Product Now In Distribution
CannAmide, a pharmaceutical drug for use as an anti-inflammatory to help relieve chronic pain, is the first of SciSparc's products to be commercialized. The product:
- consists of a proprietary palmitoylethanolamide (PEA) oral tablet formulation,
- is produced in a GMP facility under a product license from Health Canada's Natural and Non-prescription Health Products Directorate,
- is only available in Canada and
- is currently being introduced to pharmacies in Canada and will be expanded into other retail outlets across Canada as soon as distribution agreements can be arranged.
Management Commentary
Adi Zuloff-Shani, PhD. the Company's Chief Technology Officer, is confident that:
- CannAmide is the first of further product launches and that,
- SciSprac can fully leverage the medical and financial potential of its growing technology and product pipeline.
Amitai Weiss, the company's Chief Executive Officer, is especially enthusiastic regarding opportunities within the medical cannabis sector, namely:
- the recent announcement of the $7.2 billion acquisition of GW Pharmaceuticals (GWPH), by Jazz Pharmaceuticals, as well as
- the possibility that cannabis will be legalized by the U.S. federal government under the Biden administration
And that SciSparc is well-positioned for success going forward given that it has a number of products that are in a preparation state for clinical trials.
SciSparc's Drug Development Programs
SciSparc is currently engaged in the following drug development programs based on tetrahydrocannabinol (THC) and/or non-psychoactive cannabidiol (CBD):
- SCI-110 is an innovative and proprietary drug candidate platform based on Delta-9-Tetrahydrocannabinol (THC) and Palmitoylethanolamide (PEA), for the treatment of central nervous system diseases such as agitation in Alzheimer's, Tourette Syndrome, and Obstructive Sleep Apnea.
- The company has already signed an agreement with The Israeli Medical Center for Alzheimer's to conduct a phase IIa clinical trial to evaluate the safety, tolerability, and efficacy of SCI-110 in patients with Alzheimer's;
- SCI-160 is an innovative and proprietary CB2 receptor agonist intended for the treatment of pain;
- SCI-210 is a formulation of cannabidiol (CBD) and PEA for treating symptoms of Autism Spectrum Disorder and epilepsy which has already demonstrated its efficacy as compared to the effect of CBD alone and its potential of reducing adverse effects associated with high dose CBD-rich treatments.
Details of SciSparc's $8.15M Financing
To help finance its intentions the company has just closed its sale of 1,152,628 Units (each unit consist of 1 American Depositary Share, 1 Series A Warrant, and ½ Series B Warrant) to certain institutional and accredited investors in a private placement at an offering price of $7.07 per Unit for aggregate gross proceeds of $8.15M.
- The Series A Warrants and Series B Warrants have exercise prices of $7.07 and $10.60, respectively, and:
- are allowed to be exercised 6 months after issuance if the above strike prices have been reached by that date,
- and expire 5-years from the date of issue if not exercised by that date.
- The securities:
- were sold in a private placement,
- have not been registered under the Securities Act of 1933, as amended, and
- may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- The Company has agreed to file a resale registration statement with the Securities and Exchange Commission, for purposes of registering the resale of the securities issued or issuable in connection with the offering.
SciSparc's Rebranding Efforts
Starting in February the company began rebranding itself by:
- changing its name from Therapix Biosciences Ltd. to SciSparc Ltd.,
- creating a new logo (see above),
- beginning to trade under its new symbol, SPRCY,
- obtaining a new CUSIP number,
- and developing a new website. (Their current website, www.therapixbio.com, is still available to visit.)
SciSparc's Revenue Potential
As mentioned above, SciSparc's cannabinoid-based drug development programs are targeting the treatment of Tourette Syndrome, Autism Spectrum Disorder, Epilepsy, Obstructive Sleep Apnea, Alzheimer's Disease, and pain management and each has multi-million-dollar revenue potential for SciSparc going forward.
SCI-110: Tourette's Syndrome
Tourette's syndrome is a genetic neuropsychiatric disorder, characterized by physical and vocal tics, and associated with the exclamation of obscene words or socially inappropriate remarks. Tics are repetitive and non-rhythmic movements of discrete muscle groups and are formed due to genetic mutation that disrupts the production of histamine in the brain and are primarily caused due to the disturbance in the balance of neurotransmitters in the brain, which carry nerve signals from cell to cell. Tourette syndrome also increases the risk of hyperactivity disorder or obsessive-compulsive disorder in children. Blinking, frowning, jerking, and foot stamping are some of the additional symptoms of Tourette's syndrome.
The disease:
- primarily affects children aged between 6 years and 17 years with the average onset age of 3 and 9 years;
- occurs in people from all ethnic groups;
- occurs in males 3 to 4 times more often than females;
- is twice as likely in Non-Hispanic white children tan in Hispanic and non-Hispanic black children.
The U.S. dominates the global Tourette syndrome market where, as per the data of the CDC study, 1 of every 360 (0.3%) children 6-17 years of age have received a diagnosis of TS, and that incidence rate is expected to expand in the future. Europe is the second-largest market and is expanding there owing to its high incidence rate and is also expanding in the Asia Pacific and Latin America due to increasing awareness among patients.
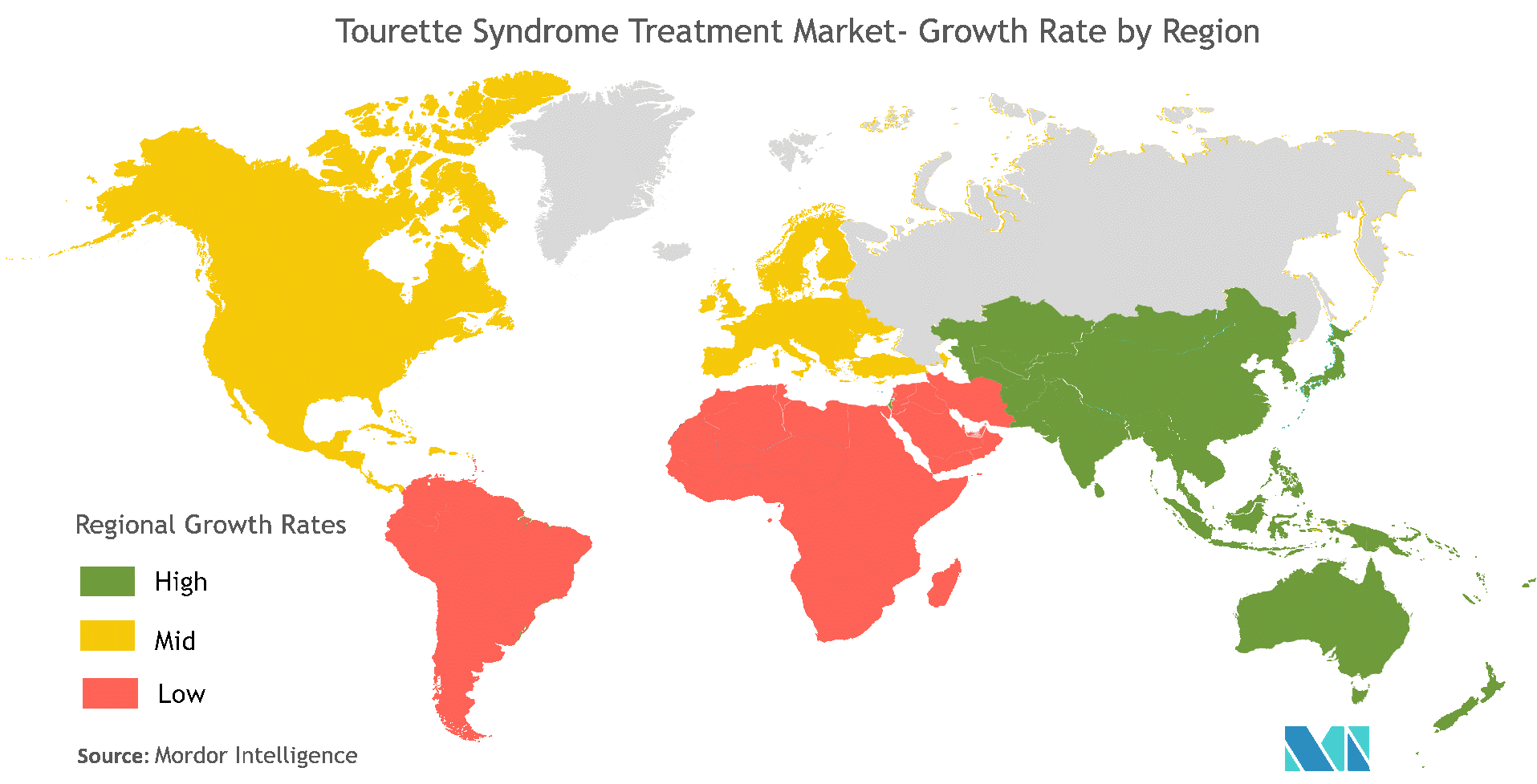
There are currently no commercially approved drugs for the treatment of Tourette's Syndrome in the market but, as mentioned above, many companies are working on the research and development of new drugs to treat the disease (e.g. AstraZeneca Plc, Boehringer Ingelheim International GmbH, Eli Lilly and Co., Mylan NV, Otsuka Holdings Co. Ltd., Pfizer Inc., Sun Pharmaceutical Industries Ltd., Neurocrine Biosciences, Inc., Novartis AG, Teva Pharmaceutical Industries Ltd.) so the drug pipeline is quite robust, and many drugs are expected to launch during the period of forecast (2020-2027).
Technavio has estimated that the global Tourette's Syndrome drugs market will progress at a CAGR of 5% ($98.77M) over the next few years suggesting that the size of the drug market is approximately $40B. Were SciSprac's SCI-110 product eventually approved by the FDA a modest 0.5% share of that market would equate to potential revenue of approximately $200M annually although such a possibility would not materialize until the end of the decade at the earliest.
SCI-110: Obstructive Sleep Apnea
Sleep apnea is a common sleep disorder that affects millions of people in the world. It takes its name from the Greek word apnea, which means "without breath." People with sleep apnea literally stop breathing repeatedly during their sleep, often for a minute or longer and as many as hundreds of times during a single night. It occurs when the muscles relax during sleep, causing soft tissue in the back of the throat to collapse and block the upper airway which may be caused by either complete obstruction of the airway (obstructive apnea) or partial obstruction which can both wake one up. Of these, obstructive sleep apnea (OSA) is the most common.
The disease is more prevalent in individuals with factors that include:
- being older,
- being male,
- being a minority race,
- having a high body mass index,
- having a large neck circumference,
- having craniofacial abnormalities,
- being menopausal, and
- having health behaviors such as smoking and alcohol use.
The standard therapy of continuous positive airway pressure (CPAP) is efficient in improving OSA and in reducing its associated comorbidities but patients often cannot tolerate CPAP, which can produce general discomfort or troublesome nasal symptoms leading to poor adherence. As such, alternative options need to be developed and pharmacological therapies are an obvious approach. Furthermore, Frost & Sullivan estimates the prevalence of OSA to be 12% or 29.4 million of the U.S. adult population with nearly 80% of the population undiagnosed which demonstrates the unmet medical need.
The global Obstructive Sleep Apnea market size was found to be US$221.8M in 2017 of which the USA accounted for 75%. The market is estimated to be increasing by 4.7% CAGR during the study period (2017–2030) which would put the market size at approximately US$400M by 2030. Were SciSprac's SCI-110 product eventually approved by the FDA a modest 0.5% share of that market would equate to potential revenue of only $2M annually although such a possibility would not materialize until the end of the decade at the earliest.
Sci-110: Alzheimer's
Alzheimer’s disease is the most common form of dementia, a progressive brain disease that slowly destroys memories and thinking skills. Symptoms usually start with difficulty remembering new information and, in advanced stages, symptoms include confusion, mood, and behavior changes, and inability to care for one’s self and perform basic life tasks. Worldwide about 50 million people have some form of dementia,
The disease:
- ranks as the third leading cause of death, after heart disease and cancer,
- affects approximately 5.7 million people in the U.S. currently (200,000 people under age 65 have early-onset Alzheimer’s disease) and that number is projected to triple to 16 million by 2050,
- doubles about every five years after age 65 and, after age 85, the risk reaches nearly 50%,
- will adversely affect Latino and African American populations as their "senior citizens" will grow 224% and 114%, respectively, by 2030, compared to a 65% growth rate for non-Latino white Americans and
- will affect African Americans twice as likely as white Americans and Latinos by 1.5 times.
According to research and consulting firm, GlobalData, disease-modifying treatments for Alzheimer’s Disease as well as novel symptomatic therapies with innovative mechanisms of action will enter the arena between now and 2030.
- Symptomatic therapies such as Lundbeck’s LuAE-58054 and Forum’s EVP-6124 are already in the pipeline and
- Biogen’s aducanumab; Eli Lilly’s solanezumab; Roche’s gantenerumab and crenezumab; Merck’s MK-8931 and AstraZeneca/Eli Lilly’s AZD-3293 are in development.
- Namenda, Aricept, and Exelon, the current AD drug market leaders, will maintain strong shares throughout the forecast period but see their combined sales fall annually with Namenda and Aricept having recently lost patent exclusivity.
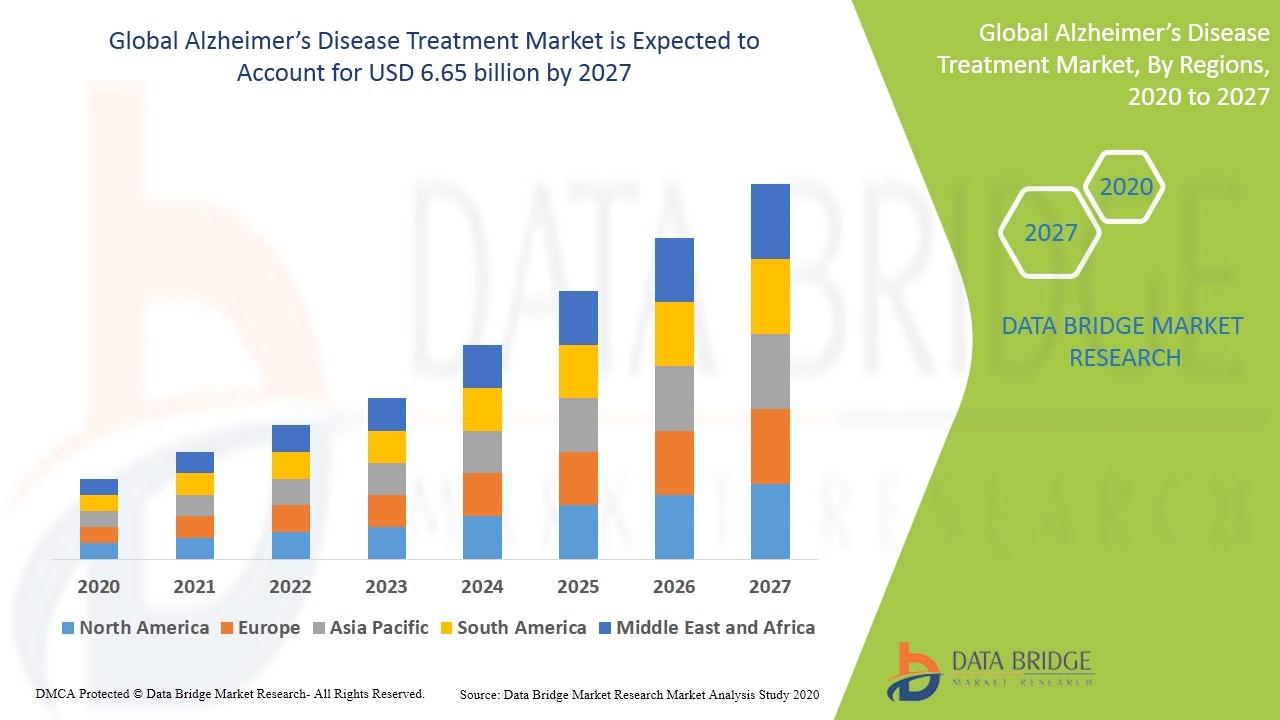
Given the aforementioned, Data Bridge Market Research expects that the Alzheimer’s disease treatment market size will grow at a CAGR of 8.29% between now and 2027 and reach US$6.65B by then. Were SciSprac's SCI-110 product eventually approved by the FDA a modest 0.5% share of that market would equate to potential revenue of $33.3M annually although such a possibility would not materialize until the end of the decade at the earliest.
SCI-160: Pain
Pain is a disturbing sensory and emotional sensation that results from tissue damage or disease ranging from acute pain for the short term to chronic pain for the long term. A variety of drugs are used to manage pain resulting from inflammation in response to tissue damage, chemical agents (pathogens), or nerve damage (neuropathic pain). Most drugs act by binding to protein targets on cell membranes and affecting biochemical processes of the body.
The rise in the geriatric population is the major factor that drives the growth of the global pain management drugs market as aged people are more prone to suffer from joint pain and other chronic conditions as well as the surge in the prevalence of chronic diseases, such as cancer, diabetic neuropathy, and osteoarthritis. In addition, the rise in the number of surgical procedures and an increase in healthcare expenditure are expected to fuel growth of the pain management drugs market.
Some of the key players operating in the market include Novartis, Eli Lilly, Abbott Laboratories, Endo Health Solutions, Purdue Pharma, Pfizer, Mylan, Merck, Johnson & Johnson, and GlaxoSmithKline Plc. The other prominent players in the value chain include Allergen, Bayer, Bristol-Myers Squibb, Valeant Pharmaceuticals, Boehringer Ingelheim, Sorrento Therapeutics, WEX Pharmaceuticals, and Zynerba Pharmaceuticals.
According to Allied Market Research, the global pain management drugs market was valued at US$71.4B in 2019 and is projected to reach US$91.6B by 2027, registering a CAGR of 3.8% from 2020 to 2027. Were SciSprac's SCI-110 product eventually approved by the FDA a modest 0.5% share of that market by SciSparc would equate to potential revenue of approximately $45.8M annually although such a possibility would not materialize until the end of the decade at the earliest.
SCI-210: Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a heritable and polygenic neurodevelopmental lifelong disorder that typically emerges as early as 12 months of age. The term “spectrum” is associated with autism disorder as the disorder shows a wide variation in the type and severity of symptoms over time.
Patients with ASD:
- face difficulties in social interaction, communication, activities, and nonverbal interactions; and
- show repetitive patterns of behaviors. ASD patients show high dependency on their routines, high sensitivity to their environment changes, or intense focus on inappropriate items presented before them.
There are 3.5 million children and adults with autism in America. with the number of autistic children rising in the U.S., at the rate of 6.2% per year. The CDC now estimates that 1 in 54 children born in the U.S. are autistic.
Given the market potential there are some major players involved, such as Johnson & Johnson Services, Inc., Bristol Myers Squibb, Otsuka Pharmaceutical Co., Ltd., Pfizer Inc, Roche Group, Allergan, Q BioMed Inc., AstraZeneca, Behavior Innovations, and Hopebridge, LLC so SciSprac has lots of company.
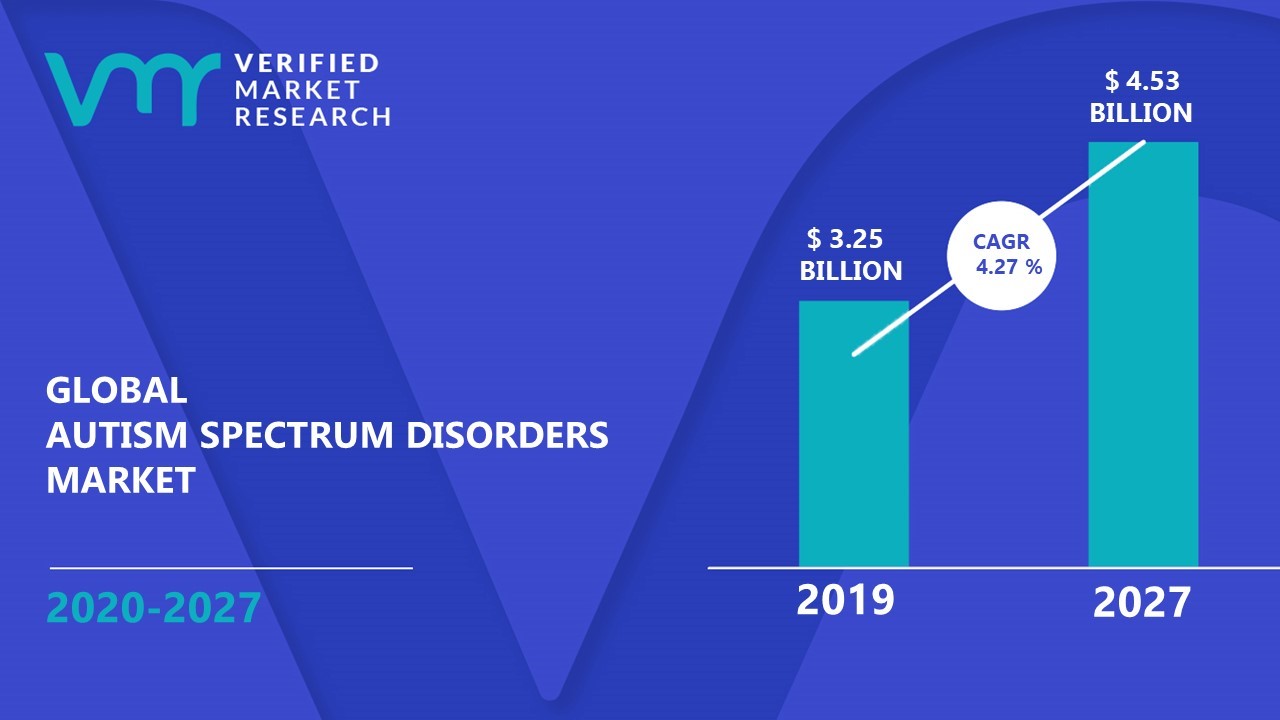
The global market for Autism Spectrum Disorder (ASD) is growing at 4.3% CAGR and should reach US$4.5B by 2026. Were SciSprac's SCI-210 product eventually approved by the FDA a modest 0.5% share of that market by SciSparc would equate to potential revenue of approximately $22.5M annually although such a possibility would not materialize until the end of the decade at the earliest.
SCI-210: Epilepsy
Epilepsy refers to a chronic neurological disorder which causes constant and unfounded seizures caused by some traumatic incident or past brain injury. The major factors driving the epilepsy treatment market are the increase in the number of accidents and brain injuries, the high rate of post-traumatic epilepsy (PTE) and post-traumatic seizure (PTS) occurrence in patients with brain injuries, and the spike in neurological disorders such as depression and Parkinson’s. The increase in funding by the government to enhance and develop effective drugs and treatments for seizures and the rise in the research and development activities to provide advanced healthcare facilities accelerate the epilepsy treatment market growth. Additionally, a growing geriatric population, a rise in awareness about the treatment options available, and price erosion of the treatments positively affect the epilepsy treatment market. According to the World Health Organization report on epilepsy, nearly 50 million have epilepsy.
According to the latest report by Market Research Future the global epilepsy market size is expanding at 8.2% CAGR and is poised to reach US$9.5B by 2023 and, if the trend continues as expected, should reach US$13B by 2027. Were SciSprac's SCI-210 product eventually approved by the FDA a modest 0.5% share of that market by SciSparc would equate to potential revenue of approximately $32.5M annually although such a possibility would not materialize until the end of the decade at the earliest.
Some major players of the epilepsy drugs market include GW Pharmaceuticals, UCB Pharma, Sanofi, Valeant Pharmaceuticals, Johnson & Johnson, Eisai Co, Abbott Laboratories, Novartis, GlaxoSmithKline, Sunovion Pharmaceuticals, and Cephalon.
In summary, assuming SciSprac's SCI-110, SCI-160 and SCI-210 drug development programs were all successful and received FDA approvals for commercialization, and assuming a modest, albeit arbitrary, 0.5% market share for each of the 6 disease treatments, the revenue potential for SciSparc could possibly reach US$336M by 2030.
Conclusion
In-depth research of the company's management and finances, products under development, diseases being addressed, the drug market sizes of each, and the companies competing for business there, suggests that SciSparc Ltd. has potential but I question the merit of investing in the company at this point in time. In spite of that, however, it belongs on your watch list. All it takes is for:
- one of its products under development to get approved by the FDA and become a blockbuster hit or
- for another up-and-coming company to acquire it.
- GW Pharmaceuticals, for example, continues to soar going up 142% in just the last 4 months (as of mid-March) with its purchase by Jazz Pharmaceuticals and
- Zenabis Global has soared by 133% in just 3 months with the announcement that it is being acquired by Hexo Corp.
Either of the possibilities would cause SciSprac's stock to soar, too, so SciSprac is for the patient investor. Either keep watch and jump in when developments dictate or buy now and hold for the foreseeable future.
Disclosure: This article is part of a new “UnderCovered” series of exclusive articles featuring companies with limited coverage. Authors are compensated by TalkMarkets for their time ...
more



Good read, thanks.
Quite a serious bit of coverage and analysis in this article. Thanks.
Agreed. Nice to see.
Sounds promising.
When will CannAmide be available outside of Canada?
Why is there nothing on SciSparc's website? Unless there is something wrong with my internet, every page is blank except for the homepage, and that is practically blank.
https://scisparc.com
Thanks for putting $SPRCY on my radar!