Matinas Expands Pipeline, Moves MAT2501 Into Phase 1
TM editors' note: This article discusses a penny stock and/or microcap. Such stocks are easily manipulated; do your own careful due diligence.
Earlier this week, Matinas BioPharma Holdings, Inc. (MTNB) announced the company commenced dosing in a Phase 1 clinical study of MAT2501, an orally administered encochleated formulation of the broad spectrum antibiotic, amikacin. The initial indication for MAT2501 is for the treatment of nontuberculous mycobacterium (NTM) infections, a potentially serious and life-threatening disease that affects nearly 90,000 individuals in the U.S.
On December 8, 2016, the company announced it has received a research contract award from Cystic Fibrosis Foundation Therapeutics Inc. (CFFT), the non-profit drug discovery and development affiliate of the Cystic Fibrosis Foundation, to study MAT2501 for the treatment of NTM in pre-clinical models of cystic fibrosis (CF). The award provided by CFFT will support a collaborative research program between Matinas and Colorado State University to study the efficacy of MAT2501 in NTM infection in a CF lung model developed by the university.
Amikacin is a powerful antibiotic with limited resistance. Unfortunately, tolerability is poor and adverse events, including severe nephrotoxicity and ototoxicity, is common. This greatly limits uptake of the drug and relegates use to last-resort indications. Additionally, Amikacin must be delivered via intramuscular or intravenous injection. Nebulized and inhaled formulations are under late-stage development, but to date, no oral formulation has been approved by the U.S. FDA. Matinas MAT2501 has important differentiating characteristics that could make the drug a tremendous commercial success. Below is a quick review of MAT2501 and why I believe the initiation of a Phase 1 study is an important milestone for Matinas.
Quick Background On Amikacin
Amikacin is an aminoglycoside antibiotic used to treat serious bacterial infections. The drug is highly potent with broad-spectrum antibiotic activity (1). Amikacin works by binding to the bacterial 30S ribosomal subunit, interfering with mRNA translation and synthesis of important proteins vital to bacterial growth and survival. Amikacin has synergistic effects with beta-lactam antibiotics and although antibiotic resistance to aminoglycosides exists and is increasing, resistance to amikacin is less common due to the drugs conserved moiety (attached to N-1) and inhibition of multiple biological mechanisms, including acetylation, phosphorylation, and adenylation (2).
The most common uses in the U.S. are in treating severe, hospital-acquired infections with multidrug-resistant Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter, and Enterobacter. Amikacin can also be used to treat non-tubercular mycobacterium (NTM) infections and tuberculosis (if caused by sensitive strains) when first-line drugs fail to control the infection. NTM is the initial focus for Matinas with MAT2501.
Despite powerful efficacy and low resistance, Amikacin use in the U.S. is limited due to severe nephrotoxicity (3). Drug-induced acute kidney injury with amikacin is common, with roughly 20% incidence, and exacerbated by the presence of previous kidney damage, diabetes, and hypertension (4). Because of this risk, blood levels of the drug and markers of kidney function (i.e. creatinine) should be monitored and dose adjusted if necessary. Ototoxicity (hearing loss) is another remarkably significant side effect of amikacin use, with incidence rates found to be as high as 70% in some studies (5). The toxic side-effect profile of the drug relegates its use to a position as an antibiotic of last resort.
Amikacin may be administered 1-3 times a day but is only available as an intravenous or intramuscular injection or via nebulization. There is no oral formulation available as amikacin is not absorbed orally in its free form. In people with kidney disease, dosage must be adjusted according to the creatinine clearance, usually by reducing the dosing frequency. An orally bioavailable formulation of amikacin that improves ease of administration and reduces nephrotoxicity would be highly desirable to the infectious disease medical community.
Matinas Cochleate Technology
MAT2501 utilizes its cochleates technology to encapsulate and protect amikacin so that the drug can be dosed orally. Cochleates are stable, crystalline phospholipid-cation delivery vehicles that are composed of soybean phosphatidylserine (PS) and calcium. Calcium is used to create a calcium-phospholipid anhydrous crystal structure that traps the active pharmaceutical ingredient (API) inside the nano-crystalline structure. Once formed, the compound has a unique multilayered structure and no internal aqueous space. Cochleate drugs can be taken orally because the high calcium concentration inside the digestive tract keeps the cochleate structure in crystalline form. This protects the API even though the outer layers of a cochleate crystal may be exposed to harsh environmental conditions or enzymes found in the digestive tract.
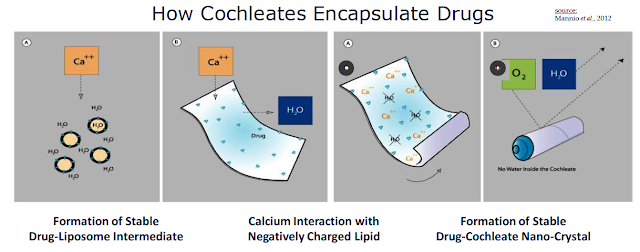
"Encochleated" molecules within the interior of the cochleate structure remain intact resulting in an increase of oral bioavailability, lower toxicity, and the opportunity for intracellular targeting. The arrangement of a drug product inside the cochleate elongated shape creates a tightly packed bilayer. Macrophages readily engulf cochleate through phagocytosis. The vesicles form endosomes, which merge into lysosomes inside the macrophage.
Once inside the macrophage, the nano-cochleate structure unrolls due to the low calcium concentration of the cytoplasm. This releases the active pharmaceutical drug inside the macrophage. The macrophage then travels to the site of the infection, slowly releasing the active anti-infectant where it is needed most.

Management refers to the cochleate-mediated delivery of API as the "Trojan Horse" hypothesis. The API is protected from the harsh environment of the GI tract, actively taken up by macrophages, and trafficked to the site of infection by humoral response. The lower plasma API level results in less systemic toxicity and increased efficacious concentration around the site of the infection. This is in stark contrast to the traditional model for drug delivery in which high concentrations of the API in the extracellular milieu are necessary for intracellular penetration. The conventional approach results in a relatively low percentage of circulating drug entering the cell and nonspecific toxicity.
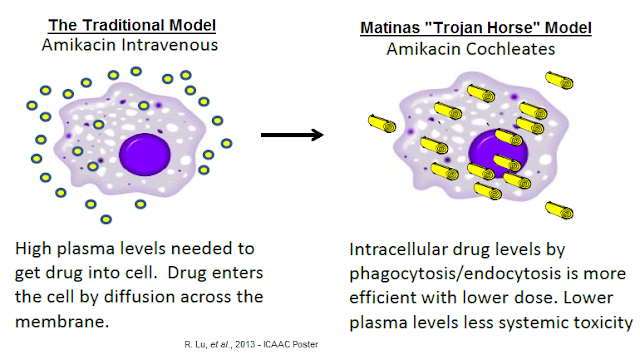
The potential to change the pharmacokinetics and biodistribution of drugs provides some significant advantages of encochleated molecules. Cochleates themselves appear to have a nontoxic profile and to improve the safety of toxic drugs. They are composed of safe products: phosphatidylserine and calcium. Phosphatidylserine is a natural component of all biological membranes and is most concentrated in the brain. Thus, there is minimal worry of adverse effects from the cochleate itself.
MAT2501 Proof-of-Concept
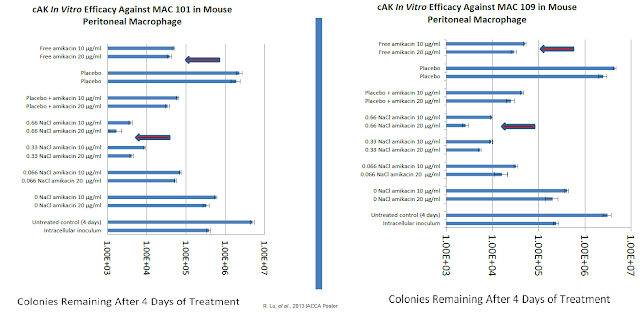
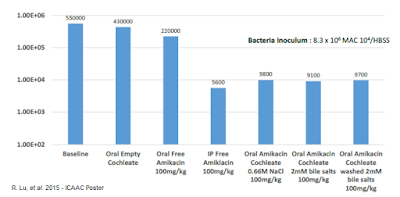
MAT2501 is a lipic-crystal nanoparticle formulation of amikacin that is currently in Phase 1 clinical studies. The in vitro activity of cochleated amikacin (cAK) was presented at the 2013 American Society for Microbiology's Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC) scientific session. Work by company R. Lu, et al evaluated the efficacy of cAK using mouse peritoneal macrophage infected with two Mycobacterium avium complex (MAC) strains. The analysis included a positive control (free amikacin) and negative control (empty cochleate). Data show a greater than 10-fold enhanced efficacy for the optimized dose of cAK compared to free amikacin.
(Click on image to enlarge)
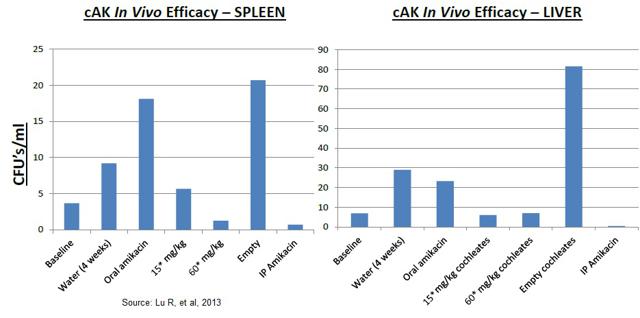
To evaluate the activity of the cAK in vivo, C57 BL/6 mice were infected with M. avium 104 by tail vein injection (disseminated NTM model). The mice were then treated with various amikacin preparations (IV free amikacin vs. oral cAK) for two weeks, after which time the mice were harvested and the bacterial load in the spleens was quantified. The results indicate that cAK was just as effective as free amikacin and far superior to orally administered amikacin or an "empty" cochleate control.
M. avium has been shown to form biofilms associated with recurrent lung infections. These infections are often resistant to conventional beta-lactam antibiotics. To study the effects of cAK in this difficult to treat infection, the company studied cAK vs. free amikacin (IV) and empty cochleates in polarized A549 alveolar epithelial cells. Encochleated amikacin showed significant activity against M. avium in the in vitro biofilm model and the in vivo respiratory biofilm mouse model. These data were presented at ICAAC in September 2015.
(Click on image to enlarge)
Phase 1 Study In NTM Initiated
On December 5, 2016, Matinas announced they have commenced dosing in a Phase 1 clinical study of MAT2501, with an initial target indication of nontuberculous mycobacterium infections (NTM). The Phase 1 study is a double-blind, placebo-controlled, single ascending dose study to evaluate the safety, tolerability, and pharmacokinetics of MAT2501 in healthy adult subjects. The primary objectives of the study are to assess the pharmacokinetic (PK) profile of amikacin following single ascending oral doses of MAT2501 as well as safety and tolerability. Secondary objectives include the assessment of the effect of food on the PK of amikacin following a single oral dose of MAT2501.
The company also intends to explore the development of MAT2501 for the treatment of a variety of multi-drug resistant, bacterial infections, including Enterobacteria, Acinetobacter, Pseudomonas, Staphylococcus aureus, and other gram-negative infections. Results from the Phase 1 PK study are expected during the first half of 2017.
NTM - A Large Market Opportunity
Nontuberculous mycobacteria (NTM) are naturally occurring organisms found in water, soil, plants and animals. While NTM infections are predominantly minor events for healthy individuals, for persons with preexisting diseases such as COPD, emphysema, bronchiectasis, and cystic fibrosis, the infections are often serious and life-threatening. Complications include pulmonary disease, skin and soft tissue disease, joint infections and, in immunocompromised individuals with HIV or AIDS, disseminated infection.
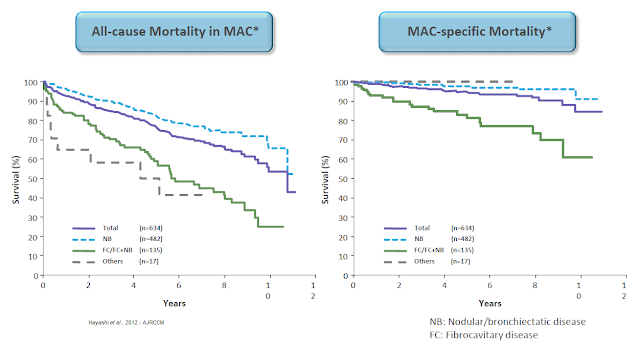
NTM infections in the U.S. are rare; diagnosed cases are estimated between 50,000 to 100,000 cases per year in the U.S. However, NTM is often under-diagnosed or misdiagnosed. NIH estimates peg the actual number of infections in the U.S. at closer to 200,000, predominantly in elderly individuals living in the coastal and gulf states (6). In persons with compromised lung function and/or immune deficiency, NTM infections can become chronic and progressively destructive. Conventional antibiotics are often ineffective due to resistance, leading to high costs of care (7).
And high mortality rates for subjects with nodular / bronchiectatic or fibrocavitary disease (8).
(Click on image to enlarge)
FDA guidance for the treatment of patients with of NTM infections refractory to guideline therapy includes a treatment duration in the range of 12 to 18 months. The efficacy of amikacin in NTM is well documented in the literature. A recent study published in Respirology by a team of researchers out of Australia found that amikacin is highly effective in treating NTM, but that the risks of toxicities must be heavily weighed for each patient (9). Inhaled formulations of amikacin, like the one under development at Insmed Inc. (INSM), has shown mixed results (10, 11), and independent studies note common toxicities, including nephrotoxicity, ototoxicity, declining lung function, hemoptysis, and dysphonia (12).
Matinas has designed MAT2501 to allow for safe and tolerable long-term use of amikacin treatment. I see MAT2501 as a significant market opportunity for the company, perhaps on par with the $1.25 billion I believe is the peak sales opportunity for Matina's Phase 2 oral encochleated amphotericin B candidate, MAT2203 (read more about MAT2203). Up to 25% of all treated NTM patients are resistant to conventional antibiotics according to LEK Consulting, putting the potential target population in the U.S. at 10-15,000. With pricing comparable to other branded, long-term use antibiotics, the market opportunity in the U.S. is $650 million (estimated at $1.0 billion WW).
The U.S. FDA has already designated MAT2501 as a Qualified Infectious Disease Product (QIDP) and as an Orphan Drug for the treatment of NTM. If MAT2501 is ultimately approved by the FDA, the seven-year period of marketing exclusivity from orphan designation combined with the additional five years of marketing exclusivity provided by the QIDP designation, provides for a potential total of 12 years of marketing exclusivity.
Conclusion
I am particularly excited about the initiation of the MAT2501 Phase 1 program. I believe Matinas BioPharma is vastly undervalued at today's level of only $85 million and my financial model only includes MAT2203 at this stage. My valuation for MAT2203 is $400 million, and MAT2501 targets an equally large market opportunity similar to MAT2203. The obvious direct comparison for MAT2501 is Insmed's Arikayce™, currently in Phase 3 clinical trials. Analysts that cover Insmed peg sales of Arikayce between $300 and $800 million (data on file).
Arikayce is an inhaled formulation of amikacin for lung infections. Matinas' MAT2501 has broader potential use outside of the lung with a significantly reduced risk of pulmonary complications. Insmed has no other clinical-stage candidates in development (see INSM pipeline), making the company's market value of $815 million truly eye-opening for investors in Matinas BioPharma. Insmed is worth nearly 10x Matinas and Matinas had MAT2203 (oral AmpB) in Phase 2 clinical studies. The initiation of the MAT2501 Phase 1 program makes my $3.50 target price on Matinas look conservative. Phase 1 data are expected during the first half of 2017 on MAT2501. Data on three separate Phase 2 studies with MAT2203 are expected throughout 2017, with data from the recently initiated VVC study expected around the middle of the year.
Disclosure: Please see important information about BioNap and our relationship with MTNB in our Disclaimer.
more



