Finally A Real Cancer Diagnosis Breakthrough?
Todos Medical is a solid diagnostics company that utilizes blood tests for the early detection of cancer and neurodegenerative diseases. But is it a good investment? I took a deeper look and reached out to the company's CEO, Herman Weiss, to get the facts.
Todos Medical (TOMDF) has already made significant progress in multiple areas of interest. These include breast cancer screening and colorectal cancer screening. In addition, it was able to sign an agreement last year with Amarantus Biosciences (AMBS) to develop the LymPro Test 2.0. Matter of fact, this test recently reported impressive findings from one study known as LymPro PET 1. This is a promising speculative in-vitro diagnostics company, because it is gearing up to launch a breast cancer blood test in the coming months.
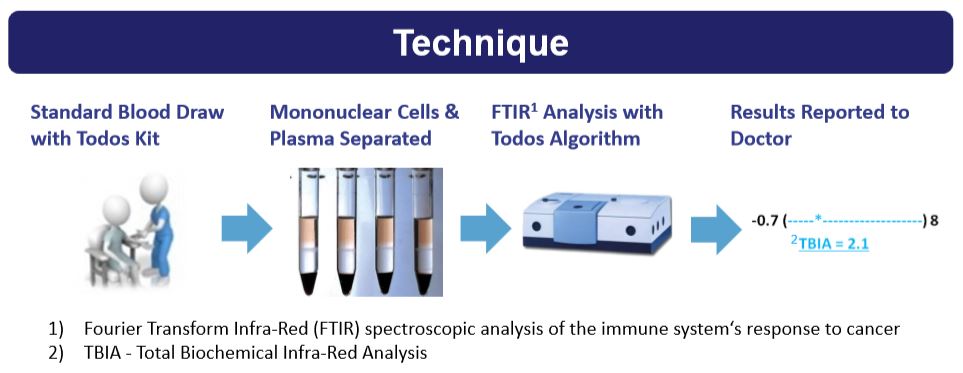
For starters, this company has a very innovative technology in place for blood tests. That’s because it has been able to develop something known as TBIA platform. TBIA stands for “Total Biochemical Infrared Analysis”. I believe that this biotech is onto something very special with this technology. That’s because other diagnostic companies for cancer screening use their technology to detect circulating malignant DNA. What that means is that their screening looks for cancer or biomarkers of tumors themselves. The problem with a blood test like that is that tumors aren’t detected until the cancer is already in the advanced stages. Well, Todos is taking another approach. Instead, it is looking to detect how the immune system reacts to tumors inside the body through a simple blood test. This is accomplished by looking at biochemical changes in plasma and peripheral blood mononuclear cells ((PBMCS)). The advantage of this type of method, is that a person’s cancer can be detected at a much earlier time frame. That’s not the only advantage in place. Other advantages of the TBIA platform are: Lower costs to produce, highly patient compliant, and easier to scale in terms of manufacturing.
Most impressive of all, the blood screening cancer test offered by Todos only requires 10 CCs (cubic centimeters) of a patient’s blood. That’s very little in the grand scheme of things. On average, a person has about 5,000 CCs of blood in their body. As you can see, only needing a blood draw of 10 CCs for a cancer screening test is very attractive. The beauty of TBIA technology is that it can still detect different types of cancer, despite each one affecting the immune system in a different manner. The best part of all is that the TBIA platform has already completed multiple studies, spanning about 1,613 patients in total. Cancers that were being detected were: Leukemia, breast cancer, colon cancer, and many other types of cancer.
The most notable item to talk about is the breast cancer screening tests known as TMB1 and TMB2. That’s because both of these products have been CE marked. That means they have gained authorization to be marketed in the European Union. Todos expects to start out by commercializing the TMB2 blood test for breast cancer screening first. That’s because it had made a deal with another company known as Orot+. The deal was made so that both companies could start to generate sales for TMB2 in both Romania and Austria. Considering that the test was CE marked, other countries in the European territories will likely follow over time. There are multiple fronts to get the product marketed in Romania and Austria. There is ongoing training, and a test is currently underway with 50 to 100 patients. Pilot blood tests are expected to start in Q2 of 2019. If all efforts go smoothly, then it’s possible that commercialization could happen by Q4 of 2019. This is an exciting step in my opinion. That’s because data generated from these efforts could be used in parallel to gain FDA approval also which is what Todos intends to do. The best part is that Orot+ will be responsible for the marketing and distribution of the breast cancer tests in the Romanian and Austrian regions.
Just as I noted above, the TBIA platform can be applied to other types of cancer. However, Todos has to generate the right algorithm for each target indication that is being screened. That’s why the company is also in the process of getting its colon cancer test ((TMC1)) to market as well. However, this type of test is not expected to be commercialized until Q2 of 2020. That’s still a bit of a way off, but for the time being, the TMB2 test being marketed in Romania and Austria for breast cancer screening is a good start. There is even going to be efforts to get this test to Israel as well for commercialization. The eventual goal is to reach as many territories as possible. Even efforts to get the cancer tests CLIA certified in the U.S. is underway.
The role of the immune system and being able to detect cancer at an earlier stage is impressive. To further this research, Todos Medical chose to close a joint venture transaction with Amarantus BioScience). Todos Medical issued to Amarantus 19.99% of the outstanding ordinary shares of Todos, in exchange for 19.99% of Amarantus’ wholly-owned subsidiary known as Breakthrough Diagnostics, Incorporated. This is just the first part of the equation in terms of this deal. That’s because Todos Medical will have an exclusive option to acquire the remaining 80.01% of Breakthrough Diagnostics in exchange for an additional of 30.01% of Todos’ shares. This is important because Todos gained the rights to LymPro, which is an immune-based blood test to detect Alzheimer’s disease. This transaction was made possible as a result of Todos’s success in raising $1,350,500 from private investors.
I believe that this deal is substantial; it broadens the scope of applying an immune-based blood test for another target besides cancer. The Alzheimer’s market is a large one, where medical costs exceed $250 billion. It is estimated that this cost could reach $1.2 trillion by the year 2050. There are currently about 5.2 million Americans with Alzheimer’s disease. At least 500,000 more people are diagnosed with Alzheimer’s each year.
A test like LymPro 2.0 could be used to detect Alzheimer’s at a more proficient rate. Current detection is around 70% for Alzheimer’s tests, but Lympro 2.0 might be able to achieve 90%. Such an improvement would not only serve to help patients with the disease, but it will give an upper hand to big pharmaceutical companies. The reason why is because it would allow big pharmas to recruit far more accurately for clinical studies. It would also cut patient acquisition costs by 50%. Think about how valuable such a blood test could be for a pharmaceutical company. The current failure rate for producing an Alzheimer’s drug is 99%. With a big pharma using this blood test it could reduce the risk of recruiting patients who don’t have AD at all and reduce costs for trial recruitment as well.
There is a profound effect for Todos Medical as well. It would validate the blood test for the company and would definitely make it easier to receive CLIA certification for it. Lastly, Todos Medical might be able to produce a collaboration agreement, whereby it could receive a large sum of an upfront payment in exchange for the use of this blood test. That would help fund the other blood tests in its arsenal. In other words, it would knock out multiple birds with one stone. Pilot studies for the LymPro blood test are expected to begin in 2020 for full approval. The goal for this AD blood test would be the same as the cancer blood tests noted above, which is to get CE mark to market it in the European territories as well.
Todos Medical is a good diagnostic company to consider for your portfolio. It is on the verge of getting multiple blood tests to market between 2019-2020. As of late April, Todos filed the F-1 for a Maxim-underwritten uplisting to Nasdaq. I believe this was a smart move for the company, because it is going to bring many new investors into the mix. Additionally, it will bring in more eyeballs to this in-vitro diagnostic company which is looking to detect cancer and neurodegenerative diseases at an earlier stage.
Investors won’t be without any catalysts either. There are plenty on the horizon. For instance, the breast cancer test is going to be commercialized in Europe and Israel in the coming months. On top of that, Todos is looking to commercialize its colon cancer blood test in Q2 of 2020. Such events for commercialization should get some sales going, while other assay validation takes place to push for approval for other cancer indications. As I noted above, the TBIA platform can be applied to other types of cancer. That means other potential catalysts would be the filing of new patents based on new tests being implemented for other types of cancer. There will likely be studies released from time to time, for several of the other blood tests currently in the pipeline. I believe such events could possibly cause Todos’ stock to trade higher. The most notable catalyst would be the exclusive option of acquiring the remaining 80.01% of Breakthrough Diagnostics. I believe such an event would reignite investor sentiment for the company.
Disclosure: This article is part of a new “UnderCovered” series of exclusive articles featuring companies with limited coverage. Authors are compensated by TalkMarkets for their time, and ...
more



Immunology and diagnostic is an interesting phase in the diagnosis of cancer! The future and answers to diagnosis and treatment of cancer lies in the various aspects of immunology. RBNbHealthcare.com is also offering immunology therapeutics as a treatment option for advanced cancers! Hope to see good results for the research! https://rbnbhealthcare.com
Any updates to this article?
This sounds very encouraging. I'll be keeping an eye on $TOMDF.
Very impressive results.
I find this kind of stuff really fascinating.
Yes the science of it is quite interesting.
For those of us who aren't big in this sector - can someone sum this up for us lay investors? I don't need to understand the science - this place doesn't publish fake news. But what does it all mean in simple terms?
basically it's a cheaper blood test and it can detect cancer at an earlier stage than other tests. That's because it doesn't look at detecting cancer itself, it detects how your immune system reacts to the cancer.
Can this test work with "cold" immune systems? By "cold" I mean an immune system that doesn't appear to pick up on malignant cells that would normally be terminated. It occurs a lot in melanoma patients.
Good question Brian.
Hi Brian, There is a point in time when the immune system does indeed react to the malignant change and shift from tumor suppressive to tumor tolerant and there is an interaction that the tumor 'turns off' the host reaction to fight it, we have shown data indicating we can still pick up these changes.
Impressive.
Thanks. Do you know if any competitors are developing anything similar?
There are competitors looking at circulating DNA from tumor cells, but this usually happens later in the disease process and not early
Big news.
yes good for both investors and for shareholders of the company. Will start to improve as more territories are commercialized.
As a socially responsible investor, this company is very appealing. $TOMDF
It is quite interesting, if they can pull it off in good fashion commercially they have a shot to do great things.
Great news for both investors and cancer patients.
Yes it will be good in the future to detect cancer at an earlier stage.
Terry/Herman, how did you evaluate the Lympro2.0 PET 1 data? I haven't seen that publicly released. And where did you see it could be as high as 90%? That would be great if it was, is that sens/spec or what? What are have KOLs or potential industry partners set as diagnostic goalposts?
Chuck, i want to add, the quote of 'could be as high is 90%' was clearly aspirational based on targets that have been developed with pharma in mind, to your second question.
This question seems suspiciously unanswered.
Sorry David, i have been traveling, please see my answer
Thanks @[Herman Weiss](user:94004). I'm impressed without how responsive you are to users' questions.
Hi Chuck, Just logged in for the first time since this went live, and seeing some great feedback. We are putting together our data package, but need to take care of a few things before we can publicise it. The KOLs we have canvased and the Pharma companies have all told us that any diagnostic that pushes the diagnosis earlier will be highly sought after
@[Herman Weiss](user:94004) @[Terry Chrisomalis](user:5023)
I'd like to know this as well.
Harry, Our KOLs and Pharma contacts are all eager as well
A real shame @[Herman Weiss](user:94004) or @[Terry Chrisomalis](user:5023) won't answer this. You've had almost a week fellas. Seems to say it all....
They did answer you Chuck.
Sorry for the delay Chuck, no trying to be evasive, I was traveling for the last 2 weeks, There was a conference in Vegas and followed by investor meetings in NYC. To directly answer your question we are working on getting the data out publicly in the right time and forum. There is a process here we are trying to be cognisant of and stay within the lines.
Dr. @[Herman Weiss](user:94004), I appreciate your reply and hope the roadshow went well. Please forgive a squeaky wheel looking for grease. A few follow-ups:
1. Did Todos/Amarantus/Breakthrough apply for the ADDF/Gates Diagnostic Accelerator? 1b. Did that application include any PET comparison data? 1c. If not, why?
2. Are you saying KOLs and potential clinical partners have not given you any firm metrics to meet? 2b. From your second response am I to take it the firm would be pleased with approx 90%s/s? Given the prior history of this diagnostic, the market would appreciate clarity and disciplined talk when it comes to efficacy.
3. Did you have the Lympro PET1 data in hand before you finalized the acquisition/partnership?
herman.w@todosmedical.com
Chuck, all excellent questions, but I would need to sign an NDA before diving deeper into the answers, happy to do so. Please reach out herman.w@todosmedical.com
Nice!
Loading comments, please wait...