BriaCell Therapeutics' Mission: Destroy Breast Cancer

Image Source: BriaCell.com
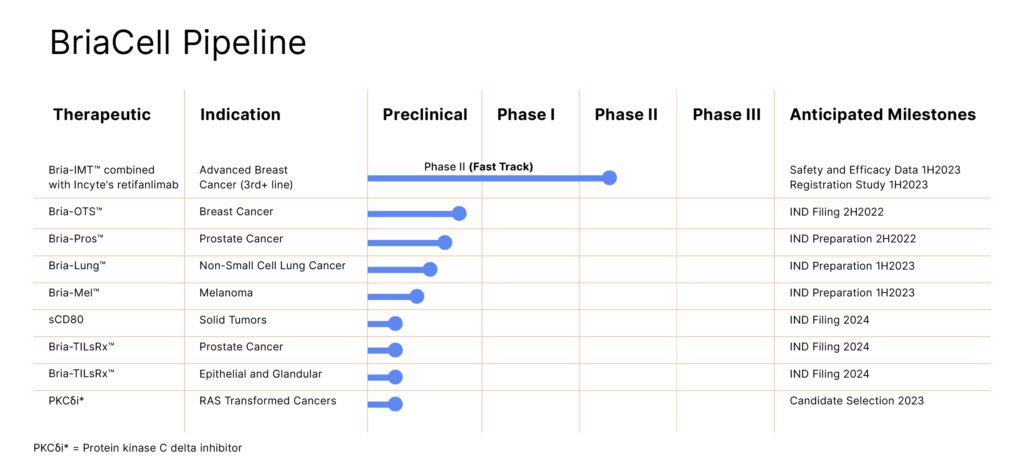
BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW|TSX: BCT) is a clinical-stage biotechnology company specializing in targeted novel immuno-therapies to fight breast and prostate cancer and also is in the early stages of researching similar therapies for non-small cell lung cancer and melanoma.
Immunotherapies have become the forefront of the cancer treatments because they use the body’s immune system to destroy cancerous tumors by boosting the body’s own cancer fighting cells, offer the potential for higher levels of safety and efficacy than chemotherapy, and may also prevent cancer recurrence.
Metastatic Breast Cancer
Advanced metastatic breast cancer (MBC) remains one of the most difficult cancers to treat and there is an urgent, unmet medical need to find well-tolerated and effective treatments for these gravely ill cancer patients who have only months to live and cannot tolerate the harsh side effects of other cancer treatments.
Bria-IMT™
That being said, BriaCell's clinical trial collaboration with Incyte Corporation (Nasdaq:INCY) to evaluate the effects of various combinations of novel clinical candidates has shown, according to Mayo Clinic Professor and Principal Clinical Investigator Saranya Chumsri, M.D., that a Bria-IMT™ in combination with retifanlimab (INCMGA00012), an anti-PD-1 antibody provided by Incyte, did not have any theoretical cross-resistance or overlapping toxicity with other MBC treatments allowing many patients to stay in the trial longer than their last therapy, suggesting the Bria-IMT™ is both well tolerated and clinically effective.
In a meeting with the FDA in the past few days the FDA has agreed on the registration study design and the primary end point. This pivotal registration study will enroll advanced metastatic breast cancer patients for whom no approved treatment options exists and its success could lead to a Biologics License Application (BLA) submission for the approval of the combination regimen for commercialization in advanced metastatic breast cancer.
Dr. William V. Williams, BriaCell’s President and CEO, reports that, “The importance of this milestone speaks for itself and is yet another major step towards our goal to become one of the leading immuno-oncology companies. Jumping directly into a pivotal study has greatly advanced our lead clinical program timetable with the ultimate goal of commercializing our novel immunotherapy approach for women with no approved treatment options.”
In addition, BriaCell is developing:
Bria-OTS™
- Bria-OTS™, the first “off-the-shelf” personalized immunotherapy for the treatment of advanced stage breast cancer has recently been awarded Fast Track status by the U.S. Food and Drug Administration.
- In addition, a small molecule program consisting of novel selective protein kinase C delta inhibitors have shown activity in pre-clinical models of several cancers and fibrotic diseases.
How Bria-IMT™ and Bria-OTS™ Destroy Cancer Cells - A Video
About Breast Cancer
- The American Cancer Society's 2022 fact sheet estimated that:
- 3.1 million women in the U.S. had a history of invasive breast cancer in 2022 and that it would reach 4 million by 2024,
- 287,850 women and 2,710 men (representing just 0.9% of the total of which the author of this article is one) are expected to be diagnosed with invasive breast cancer in 2022 in the U.S. alone and
- 43,780 (43,250 women, 530 men) are estimated to have died from breast cancer in 2022 making it the second leading cause of cancer death in women (after lung cancer).
- Approximately 62% of breast cancer cases are detected at the early stages (i.e., the cancer is found in the breast tissue, and has not spread to lymph nodes or other regions of the body), for which the 5-year survival rate is estimated to be 99%. As soon as the cancer spreads to lymph nodes under the arm or nearby tissues (regional stage), the 5-year survival rate drops to 85%.
- Once the cancer spreads to other lymph nodes or body parts (distant stage or metastatic cancer), the 5-year survival rate falls to as low as 27% (i.e. 73% of the patients die within 5 years). This shows the significant need for effective treatments for this deadly cancer.
Bria-Pros™
In addition to metastatic breast cancer, BriaCell has been developing Bria-Pros™, an off-the-shelf personalized immunotherapy for advanced prostate cancer, and has entered into a manufacturing service agreement with Waisman Biomanufacturing at the University of Wisconsin–Madison to manufacture Bria-Pros™ for anticipated use in clinical studies.
BriaCell’s Phase I/II trial in prostate cancer is expected to follow upon the completion of the manufacturing, testing, and the related regulatory filings.
4Q 2022 Financial Results
BriaCell Therapeutics is a clinical-stage startup with a market capitalization of only $87.07M. It is still in the product research and development of novel compounds and has no products and no revenue and requires a great deal of money to fund their R&D expenses. For a detailed look at all the financial metrics related to BriaCell in its last quarter (Q4 ending October 31st, 2022) and for every year going back to 2018 go here.
Summary highlights for Q4 showed:
- a SG&A expense of $7.18M attributed to the resumption of clinical trials and increased laboratory activity, including the hiring of additional employees;
- a net operating cash flow of $(4.1)M;
- growth in net operating cash flow of 22.6%;
- EBITDA of $(7.18)M;
- EBITDA growth of -17.1%;
- a net loss of $ (26.8)M or $(1.73) per share and
- cash and equivalents on hand of $51.1M.
Conclusion
From my analysis of BriaCell Therapeutics, I believe it would make a great buy and hold investment. Where else can one find an investment with the potential of treating such a huge number of gravely ill cancer patients? If, and when, it is bought out by a major pharmaceutical company, or brings a product directly to market itself, BriaCell's stock price should go up dramatically. The only downside I see in its future is that it runs out of money to continue its research, but CEO Williams, in an interview with TalkMarkets contributor Kate Hayden (see here), says they have a sufficient cash runway through the next 2 years to advance the company through its key value-creating clinical milestones.
Related Articles:
One-On-One With BriaCell Therapeutics Corp. Management
BriaCell Announces Positive End Of Phase II Meeting With The FDA For Bria-IMT(TM) Combination In Advanced Metastatic Breast Cancer
More By This Author:
The 5 Cannabis Category Indices Ranged From +0.39% To -72.2% In 2022
6 Largest Psychedelic Drug Stocks Were -20% In December
Conservative Cannabis Stocks Index Only -3% In 2022
Disclosure: This article is part of TM's' “UnderCovered” series of exclusive articles featuring companies with limited coverage. Authors are compensated by TalkMarkets ...
more



I'm bullish on this company.

It's always important to invest in stocks that also have a positive impact on the world.
I'm in. Bullish on $BCTX
Excellent read.
$BCTX is an exciting company, with a great pipeline and a ton of potential.
Impressive.
When small companies like this come along that are traded publicly it can offer a great ride for investors who want to experience the thrill of venture investing. Not for the faint of heart and one must be prepared to lose one's entire investment. That having said if the company succeeds you will have had the opportunity to participate in one of the more altruistic benefits of capitalist enterprise. The company has an impressive list of trials in the pipeline, as do many other start-up biotech companies. One to put on the watch-list before jumping in.
Good article.
Very thorough and informative, thanks.
Loading comments, please wait...