Biotech Breakthrough Could Save Millions Of Lives And Billions Of Dollars (SPONSORED POST)
TM editors' note: This article discusses a penny stock and/or microcap. Such stocks are easily manipulated; do your own careful due diligence.
Someone dies of a stroke every 4 minutes in the United States. Globally, 15 million people suffer a stroke every year. It’s debilitating physically and financially—but one little-known company has developed a medical device that hopes to challenge that deadly statistic.
It’s been quietly developing this new technology for 10 years. Now it’s released updated preliminary clinical trial results, and signed a manufacturing deal which could make a significant impact in the medical device industry.
After a decade of painstaking development, we’re now nearing the end of the long road to validation, and for investors who understand this industry, this could be the critical juncture.
The little-known company is CVR Medical (TSX:CVM.V; OTC:CRRVF), and its potentially life-saving device is the Carotid Stenotic Scan (CSS)--a technology designed to detect stenosis within arteries, or Ischemia, which is the leading indicator of strokes.
Of the 15 million people who suffer stroke every year, some 6 million are killed, while 5 million are rendered permanently disabled, according to the World Heart Foundation.
But there has been no cost-effective way to screen for Ischemia.
So, when a biotech company offers a potential solution to help prevent the second-leading cause of death in the world--and then releases positive preliminary clinical trial results, investors listen.
Now it’s hoping to charge out of the gate and take the market by storm once it manages to gain FDA market clearance.
In the meantime, the catalysts are really lining up:
On 7 September, CVR released updated results from its preliminary clinical trial that showed forward progress for the medical device, which we’ve been watching closely for some time. You can view the results from Thomas Jefferson University HERE.
And a few weeks prior, they announced another landmark achievement when they signed a letter of intent to manufacture the CSS. The deal gives CVR state-of-the-art manufacturing capabilities with Canon Virginia, Inc (CVI). This would give CVR immediate scalability, and also adds to its credibility: It’s not the first manufacturing deal the company has sealed.
Our researchers are keeping a close eye on CVR Medical Corp. because we think this is the turning point. Here’s why:
- It’s had two fresh catalysts in three weeks in an industry that is all about validation
- It’s developing a device that meets critical demand in terms of helping to prevent one of the most debilitating and deadly diseases of our time
- It’s developing a medical device that actually makes sense to the market
- It’s been doing it quietly, without blockbuster hype, and instead painstakingly going for real validation, making it the real deal.
Here are 5 reasons to keep a close eye on CVR Medical (TSX:CVM.V; OTC:CRRVF) in the coming weeks and months:
#1 Catalysts are Lining Up, and News Flow is Gaining Momentum
First, one way to look at the CSS is to think about what 3D seismic imagery did for super quick discoveries in the oil and gas industry. This is exactly what CVR’s sensory system could do for the medical industry in terms of detecting critical stroke symptoms.
This is how it works:
CVR’s Carotid Stenotic Scan (CSS) is a tool to detect blockage within the Carotid Arteries, potentially offering patients and caregivers a device for early detection in a quick and repeatable manner.
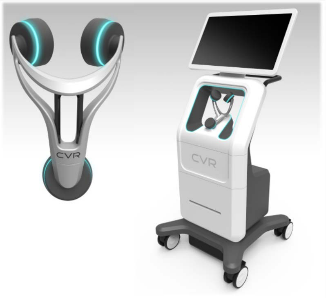
The CSS makes a connection between fluid flow and low frequency sound waves to detect arterial disease or blockage. Blood flowing through the carotid arteries produces wave patterns which are shaped and altered by the presence of irregularities on the inner artery walls.
CVR’s advanced technology captures these wave patterns and analyzes them mathematically with patented algorithms. After a brief test, the analysis is complete, offering a way to potentially identify those at risk of a stroke and arming the healthcare provider with the information necessary to prevent the deadly event.
Unlike other comparative modalities, the CSS was designed to function without the assistance of a certified technician. These three facts combine to create one of the potentially biggest—and most lucrative--phenomena in recent medical equipment market history.
The updated results of the preliminary clinical trials released just now bring the benefits of this device that much closer. Not only could it potentially help prevent strokes through early detection of arterial disease, but there is a secondary benefit: if all goes according to plan, it makes testing cheap, affordable and accessible to all.
#2 CSS Makes Market Sense at $49,000 Per Unit
Detecting the early signs of a stroke—before it’s too late—is a major challenge for our medical establishments. Often diagnoses are wrong. Usually, they are expensive.
CVR Medical’s (TSX:CVM.V; OTC:CRRVF) device, in late phase clinical trials, is expected to cost an estimated $49,000 per unit for detecting Ischemia, again—the leading indicator of stroke.
CT scans are the most common method of diagnosing a stroke—but usually after it’s already happened, and even then, stroke is not always seen on CT scans. CT scans can predict risk of stroke in patients who have suffered transient ischemic attacks (TIA), or ‘mini-strokes’, but its costs anywhere from $825 to $4800 for a CT scan, depending on your doctor and your insurance. That’s because CT scanners cost between $1 million and $2.5 million dollars each.
Coming in at the planned $49,000 per unit, compared to up to $2.5 million, CVR Medical’s CSS makes market sense. But beyond that, the CT scan isn’t enough for detection.
According to the Centers for Disease Control (CDC), early action is vital for survival, and only 38 percent of stroke sufferers even recognize they are having a stroke in time to receive effective emergency intervention.
#3 Urgent Need, Hungry Market
Few things are more urgent than an early detection system for a medical condition that kills 6 million people a year. In the U.S. alone, one American dies from stroke every 4 minutes, and right now the U.S. death toll from strokes stands at upwards of 130,000 each year. Annually, more than 795,000 Americans suffer a stroke.
“Strokes will absolutely strain the healthcare system,” says Bruce Ovbiagele, M.D., M.Sc., professor and chairman of the Department of Neurology at the Medical University of South Carolina, Charleston. Caring for survivors is expensive because stroke can cause long-term disability, he said. “Policy makers at all levels of governance should be aware of this looming crisis so that we can consider practical ways to avert it.”
Against the backdrop of these devastating statistics, CVR is hoping to make a dramatic impact upon an industry starved for innovation and advancement.
The CSS is a groundbreaking tool that can assess Carotid Arterial health in a way that has never been available to a patient, healthcare provider, or the payor in our current healthcare system.
There are 5,564 hospitals in the U.S. alone, in addition to the over 200,000 primary care physicians—many operating primary care practices, and a multitude of specialty clinics. If each relevant medical establishment purchased a single CSS device, CVR Medical would be looking at a multi-billion-dollar market opportunity at $49,000 each.
The global market, then, is gargantuan—and CVR says it’s eyeing that as well, eventually.
#4 Finally, a Solid, Sober Small-Cap Dream Team in the Medical Segment
Clinical trials are huge milestones for pharmaceutical and medical device companies, and there is nothing more important than this. More fail than succeed when it comes to clinical trials, and the winners are big.
But smart investors in this segment aren’t looking for early blockbuster potential—they’re looking for solidity, efficacy and long-term market disrupting potential.
The company’s debut medical device, the Carotid Stenotic Scan (CSS), has been quietly in development for 10 years. Instead of trying to lure in investors with early stage blockbuster talk, it waited until development was real, and the long road to validation came visibly closer to the light at the end of the tunnel.
The first thing that comes to mind when you meet the team behind CVR and the development of this breakthrough technology is an unheard-of modesty and professionalism. This team has demonstrated the strategic vision of a supergiant by leveraging intellectual property, market and industry know-how and key strategic relationships.
But what is most inviting in the tricky small-cap world of pharmaceuticals and medical devices is that this team kept its CSS development quiet until recently. There was no hype and no pump. Now they are on the solid path to real validation. This alone signals a sober solidity that makes a small-cap bet on a large-cap market attractive.
Led by Chairman, CEO and President Peter Bakema, with an impressive 30-year track record in business development, since its inception, CVR (TSX:CVM.V; OTC:CRRVF) has brought on some of the most respected medical professionals in the industry.
- Tony Robinson, COO and Executive Vice-President has been with CVR for 8 years and has extensive domestic and global healthcare experience.
- Michael Rhodes, VP of Quality Systems, is a former VP for Quality for HSBC and Motorola. He has 20 years of experience in multiple markets.
- Dr. W. Douglas Weaver, a member of the BOD Scientific Advisory Board, is the former president of the American College of Cardiology and the former VP and System Medical Director of Heart and Vascular Services at Henry Ford Health System. His over 330 publications related to drug and device discovery have been some of the most influential in our time.
Together, they are on a trajectory that plans to bring a long-sought-after solution to helping detect the risk of ischemic stroke to the market in an affordable and accessible way. Their latest news also comes at a very critical time on our nation’s—and indeed, the world’s—healthcare story.
And now, following the release of updated preliminary clinical results from Thomas Jefferson University, Bakema is ready to speak out.
“You can’t come out with a lot of changes while you are validating, so we are almost over that hurdle. We are nearly moving into pivotal trials, which puts us right at the door for FDA approval,” he stated in a recent interview.
CVR now has several clinical sites where they are initiating trials at two of the leading research hospitals in the country. Doctors at both are “excited about getting CSS in there, and we have many other sites interested in conducting additional trials” says Bakema.
After a long development journey, CVR is now becoming well-known in the medical community. The potential for research is huge, and “the sky is the limit as far as the amount of news that could come out of clinical relationships we are building now,” the CVR CEO told us.
#5 Still Priced Like Early Stage Company
CVR (TSX:CVM.V; OTC:CRRVF) has already invested US$23 million into the research and development of their breakthrough CSS technology, and right now they’re at that critical juncture where the path to potential FDA approval is at least visible.
This is a small-cap, so shares are still priced as a small-cap, but once they enter pivotal trials the window of opportunity may have closed significantly. When and if the CSS clears pivotal trials and is followed by a full clinical report, FDA submission is the next step, and then—if successful—market clearance and delivery.
Because the expected all-in manufacturing and sales costs are less than half the sale proceeds, the company is expecting a very quick and lucrative head start. The team has already lined up manufacturing and components, so once the clinical reports are in, and the FDA hurdle is cleared, sales and profits could start quickly.
They’ve also got $2 million in the bank and a number of warrants being exercised.
A key indicator of where this is going came in August, when CVR announced that after years of development, it signed a letter of intent with Canon Virginia, Inc. (CVI), a domestic manufacturing subsidiary of Canon U.S.A., Inc. The deal, when fully executed, means that Canon will manufacture the CSS at its state-of-the-art facilities. It’s a relationship that, according to Bakema, is highly important, not only for credibility, but also for scalability and reduced cost of entry to market.
Other recent manufacturing deals have also poised CVR for success right out of the gate. In March, they announced a partnership with ADCO Circuits, which will be the exclusive provider of CSS custom circuit boards for its sensors.
In combination with the recently updated preliminary clinical results, growing clinical relationships and the move towards hoped-for FDA approval, the catalysts for CVR Medical (TSX:CVM.V; OTC:CRRVF) are building—fast.
The timing couldn’t be better, for investors—or for the millions of people falling victim to stroke around the world every year.
Sources:
http://www.nytimes.com/health/guides/disease/stroke-secondary-to-carotid-stenosis/print.html?mcubz=3
http://integrisok.com/bass-baptist-health-center-enid-ok-carotid-artery-stenosis-and-strokes
https://www.world-heart-federation.org/resources/stroke/
http://www.who.int/mediacentre/factsheets/fs310.pdf
http://www.strokecenter.org/patients/stroke-diagnosis/imaging-tests/ct-scan/
http://www.medicalnewstoday.com/articles/286305.php
https://www.newchoicehealth.com/procedures/brain-ct-scan
https://www.dicardiology.com/article/costs-vs-benefits-comparing-64-slice-256-320-slice-ct
https://www.cdc.gov/stroke/facts.htm
https://www.statista.com/topics/1074/hospitals/
https://www.ahrq.gov/research/findings/factsheets/primary/pcwork1/index.html
Disclaimer: PAID ADVERTISEMENT. This communication is a paid advertisement and is not a recommendation to buy or sell securities. Oilprice.com, Advanced Media Solutions Ltd, and their owners, ...
more



Looks good.
Liked this, thanks. Bullish on $CRRVF.
This sounds very promising, but I'd be interested in seeing some more hard facts. I read the link to the actual clinical trial results but it didn't give much information other than saying that most patients responded favorably. Which obviously is a good sign but I'd like to dig deeper before I invest.
I agree, but that alone is a good sign. Looking forward to more on $CRRVF,
Nice!
Is having only $2 million in the bank for such a large company considered a good thing? Between salaries and continued R&D expenditures, I would think that's rather low. Can anyone else weigh in to let me know? Otherwise I find $CRRVF to be an intriguing prospect.
Investing aside, this is extremely promising. Anything that will help live longer is good news. I just had a friend that suffered a stroke. Here's hoping for further positive results! $CRRVF
From your mouth to God's ear.
Sounds good. What are the risks/downsides to investing in $CRRVF though?
I always take sponsored post with a grain of salt, but this is definitely worth a closer look! Any independent analysis available on this stock?
I'll echo that comment.
I've always appreciated being able to make money on an investment, while knowing that investment can help people at the same time!
This sums up everything you need to know: "So, when a biotech company offers a potential solution to help prevent the second-leading cause of death in the world--and then releases positive preliminary clinical trial results, investors listen."
I'm listening! Eager to learn more about #CVRmedical $CRRVF