VistaGen Therapeutics: Interesting Prospects
VistaGen Therapeutics (VTGN) is a biotechnology company focused on improving treatments for patients with Major Depressive Disorder (MDD). The company is taking a different approach from all other therapies that exist today. Current therapies for MDD are standard antidepressants and atypical antipsychotics. VistaGen is taking a different route with its oral drug AV-101. It doesn’t even have to steer far away in terms of clinical development. That’s because the company is using a modified version of an FDA approved drug known as Ketamine. More about Ketamine will be explained below. Just know that if the current clinical trials are successful, VistaGen can move on to other indications as well.
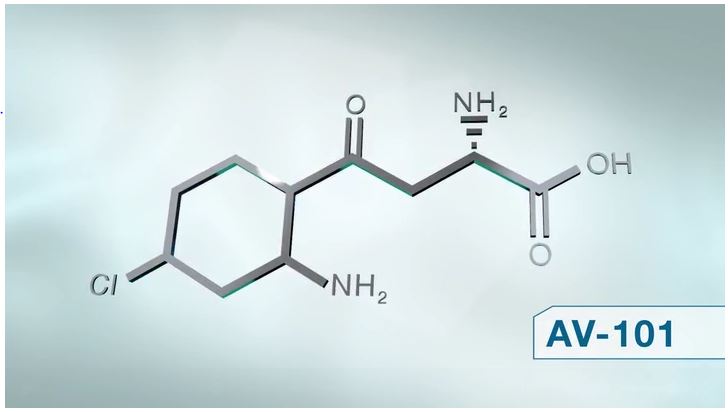
Ketamine and AV-101
There are a lot of patients who have to cycle through many antidepressants before they find the right one. Think about that for a second. These patients with MDD are in serious need of medication to help them move on with their daily lives. Having to try out multiple treatments until one works is just terrible for these patients. Even then, patients go through first-line, second-line, and third-line of treatments. Sometimes they have to take these antidepressant treatments together with atypical antipsychotics. The problem now is that many patients who just take monotherapy antidepressants go through drug resistance (when patients are immune to the drug in question or it doesn’t work for them at all).
Ketamine was approved by the FDA back in the 1970s for anesthesia. Recent studies at Yale and the National Institute of Mental Health showed that ketamine relieved treatment-resistant MDD in 71% of patients within 24 hours. However, it it not suitable for long-term use, and also requires intravenous administration. The studies catalyzed interest in development of new antidepressants that are fundamentally different from current therapies.
VistaGen's drug AV-101 is similar to ketamine in targeting NMDA and AMPA receptors in the brain. The mechanism of action of ketamine works first by entering the blood brain barrier. It targets the NMDA glutamate receptor of the brain, which then allows for the release of AMPA receptors. That’s when key connection by neurons are improved, thus improving clinical outcome. In essence, it acts through the AMPA dependent pathway. That ends up generating antidepressant effects. AV-101 intends to be safer by avoiding some of the negatives of ketamine (by inhibiting rather than blocking the NMDA receptor) and to be available in oral form.
The goal of VistaGen is not to displace people off the antidepressants they are on now. It is taking more of a combination approach where AV-101 can be combined with current antidepressants.
Ketamine Safety
VistaGen has been able to modify its AV-101 drug to act like Ketamine, but with none of the adverse side effects associated with it. That’s because Ketamine blocks ion channel activity. On the other hand, AV-101 does not block NMDA ion receptor channel activity, it inhibits it. That leads to no severe side effects for AV-101, neither like ketamine has, nor like the“black box” metabolic effects of standard antidepressants and antipsychotics.
Safety In Neuropathc Pain
VistaGen has shown AV-101 to be a safe and effective drug. The Scandinavian Journal Of Pain, a peer-review journal, published a study showing AV-101 having an excellent safety profile in neuropathic pain. There were two phase 1 studies that were performed to show the safety and efficacy of AV-101 in neuropathic pain. One phase 1 study was a single dose study, and the second phase 1 study was a multidose. The good news is that both of these phase 1 trials showed that AV-101 was very safe and well tolerated in all patients. In addition, there were no meaningful differences in adverse events between AV-101 and placebo. This safety profile is important because it accomplishes two things. First, it gives future hopes for patients to have a treatment option for neuropathic pain as a non-opioid. Secondly, it proves how safe AV-101 is as a drug. In my opinion, the good part about having such a safe drug is the ability to be able to raise the dose levels if necessary. That in turn could potentially improve efficacy outcome for newer clinical trials.
Current Antidepressants
The biggest problem with relying on current antidepressants is that they have a lot of unwanted side effects. Such side effects include: Weight gain, nausea, decreased libido, anxiety, and many others. AV-101 has been safe and does not have such terrible side effects. The current drugs can take up to 8 weeks or more to start working in MDD patients, time that these patients can’t afford to lose. The final factor is that 1 in 3 MDD patients who take current antidepressants don’t respond to treatment.
Catalysts
The current clinical trials in place will validate the efficacy of AV-101. The phase 2 monotherapy treatment for MDD using AV-101 is expected to complete by the end of 2017. That means that investors should anticipate data around the 1st half of 2018. That will be a major inflection point for the company, because it will show top-line data of AV-101 as a monotherapy. The adjunctive phase 2 data is expected to launch sometime in 2018. Data from this trial could be expected as early as 1st half 2019. The first set of data for the monotherapy study is not that far away, which makes it a major catalyst that could greatly affect the stock price.
Drug Versatility
A big advantage for any biotech company is when one drug can be applied to a multitude of indications. That is the case here with AV-101. VistaGen has stated that this drug can be applied to a multitude of Central Nervous System (CNS) indications. Such indications include: Neuropathic pain, epilepsy, Bipolar disorder, depression, and a few others. That means that the use of AV-101 does not stop with just MDD. There are many indications that Vistagen can choose to go after next. The likely indication would be the Neuropathic pain market. For starters, the neuropathic pain market is estimated to climb to $8.3 billion by 2024. I would say the most important aspect being that new treatment options are needed, because current pain medications are opioids. The problem is that there is a major opioid addiction epidemic, which leads to overdose in many patients. On top of that, 4 of 5 heroin addicts started off with opioids before they branched off to other drugs. With a successful efficacy profile proven by AV-101 for pain, things could change.
Acquisition Potential
Another massive potential for VistaGen is acquisition. There was a privately held company by the name of Naurex Inc, which was acquired by Allergan PLC for $560 million in cash. Naurex also targets MDD, targeting the pathways NMDAR/AMPA, but intravenously. Think about that for a second. Allergan ponied up money to buy this company that treats MDD using the same pathways as VistaGen, but through IV administration. If Naurex, which uses IV administration, was bought for that much, how much do you think big pharma would pay to buy a company like VistaGen with an oral form of therapy? After all, oral administration is the preferred route for treatments, mainly because it is easier access for patients instead of having to receive an IV at a medical center or hospital.
Conclusion
I believe that VistaGen is a company that should be on investors' radar. The next few years have a few major inflection points with data that could push the company to a higher valuation. The best part about the company is that it is using a different approach to treating MDD patients, unlike current antidepressants. Most of the current antidepressants target are SSRIs, or Selective serotonin reuptake inhibitors. They may have some efficacy for MDD patients but their side effects are pretty harsh. Vistagen’s AV-101 provides a major breakthrough in how MDD patients and other neurological disorders can be treated.
Disclosure: This article is part of a new “UnderCovered” series of exclusive articles featuring companies with limited coverage. Authors are compensated by TalkMarkets for their time, and ...
more



When I say I was in a different place that was when the drug was intravenous but until Wednesday very good. Regards Paul
Thanks for sharing but I'm not clear what your point is Paul.
I suffer from MDD and was admitted to a psychiatric hospital.There are two hospitals in the UK and they are testing ketamine. One is Oxford and the other One is Northampton.The Dr ask me if I would be willing to take part in the test and when you suffered with depression for many years you would do anything to get out of the big black cloud that makes life unbearable.it was amazing it was like you were in a different place,so relaxing.The trials were every Friday you would document everyday on your computer.For me it was very effective but I noted it would last until Wednesday obviously this is a trial and as time when by they would now what dose you would require.But unfortunately I was taken of the test because I was on a very strong opioid and obviously they were testing a new drug and could not take any chances as it did not have a license. Regards Paul
Your argument for acquisition sounds convincing, but I wonder if there have been any such overtures. Know if that's the case? Which companies would be the most likely to target #Vistagen? $VTGN.
Looks promising. Sounds like $VTGN should be getting more love from investors.
Definitely sounds like some interesting prospects indeed! $VTGN
Thanks @[Terry Chrisomalis](user:5023), can you explain what a "first-line, second-line, and third-line of treatment" is?
That is taking one SSRI drug for 4-8 weeks then it not working, then taking another SSRI drug for 4-8 weeks and it not working etc. Then eventually following up with adding atypical antipsychotics
Thanks Terry! Will take a closer look at $VTGN.