Sensex Trades Marginally Higher, Nifty Above 17,250; IndusInd Bank & ICICI Bank Top Gainers
Asian share markets slipped today following a mixed session on Wall Street as investors positioned their portfolios for the new year and continued to grapple with increasing global numbers of Omicron coronavirus cases.
The Nikkei plunged more than 1% before recovering, while the Shanghai Composite fell 0.8%. The Hang Seng dipped 0.9%.
In US stock markets, Wall Street indices ended mixed as a four-day rally lost steam in thin trading and investors weighed Omicron-driven travel disruptions and store closures.
The Dow Jones Industrial Average gained 0.3% while the Nasdaq Composite fell 0.6%.
Back home, Indian share markets opened on a flat note following the trend on SGX Nifty.
Benchmark indices are currently trading on a cautious note, mirroring the weak sentiment of their global counterparts.
The BSE Sensex is trading up by 181 points. Meanwhile, the NSE Nifty is trading higher by 52 points.
IndusInd Bank and ICICI Bank are among the top gainers today. Power Grid, on the other hand, is among the top losers today.
The BSE Mid Cap index is up 0.5%. The BSE Small Cap index is trading higher by 0.8%.
Sectoral indices are trading mixed with stocks in the pharma sector, banking sector and energy sector witnessing most of the buying.
Metal stocks, on the other hand, are trading in red.
Stocks from the banking and finance sector are in focus as the RBI has warned that banks' asset quality could get dented and it specifically warned that NBFC asset quality could see a further hit.
Shares of Minda Industries and ESAB India hit their 52-week highs today.
M&M is in focus today as the company's wholly-owned subsidiary, Mahindra Engineering and Chemical Products, has agreed to sell its entire stake aggregating to 49% of the paid-up equity share capital held in Mahindra Tsubaki Conveyor Systems.
The rupee is trading at 74.75 against the US$.
Gold prices are trading down by 0.1% at Rs 47,985 per 10 grams.
Meanwhile, silver prices are trading down by 0.1% at Rs 62,466 per kg.
Crude oil prices rose for a sixth consecutive session aided by strength in equities.
In the latest developments from the IPO space, clinical research organization Veeda Clinical Research has received the capital market regulator's nod to raise approximately Rs 8.3 bn.
The issue will consist of the issuance of fresh equity shares worth up to Rs 3.3 bn and an offer for sale (OFS) of Rs 5 bn by promoters and existing shareholders.
Investors participating in OFS include CX Alternative Investment Fund, Arabelle Financial Services, Bondway Investment Inc., Stevey International Corporation and Basil Private.
The company intends to utilize net proceeds from the fresh issue for repayment of the debt, funding capital expenditure, investing and funding further acquisition of subsidiary Bioneeds, funding working capital requirements and general corporate purpose.
Veeda is one of the largest independent full service clinical research organizations (CRO) in India. It specializes in the focused segment of bio availability / bio equivalence (BA/BE) studies.
To support its capabilities and to offer top-notch clinical service for novel drugs, Veeda acquired a 50.1% stake in Bangalore-based Bioneeds India, after it acquired a substantial minority stake in the company during March and July 2021.
How and when this IPO comes out remains to be seen.
In other news from the IPO space, the regulator on Tuesday tightened certain rules regarding IPOs. These include new guidelines for determining quota for high net-worth individuals and a longer lock-in period for anchor investors.
The regulator said that from April 2022, 33% of shares allocated to non-institutional investors will be reserved for investors with application sizes ranging from Rs 2 lakh to less than Rs 10 lakh. The remaining, two-thirds of the portion, will be earmarked for applicants whose application size exceeds Rs 10 lakh.
Meanwhile, for anchor investors, there will be a change in the lock-in period of the shares bought by them in the anchor issue of an IPO.
The current lock-in period of 30 days after the allotment of shares will continue for anchor investors for half of the shares allotted to them. For the remaining portion, the lock-in period has been enhanced to 90 days from the day of allotment.
Note that the above move by the watchdog comes at a time when there's a strong line-up of IPOs. With these new rules, the regulator seeks to protect retail investors after a record year of IPOs.
We will keep you updated on the latest developments from this space. Stay tuned.
Speaking of the current stock market scenario, amid the ongoing volatility, have a look at the two charts below, in the order they have been placed:
Near Term Volatility in Sensex Compensated by Long Term Gains
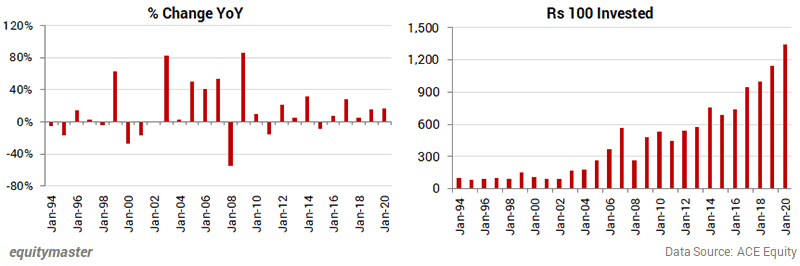
The year-on-year change in the Sensex was hardly predictable but someone who stayed invested multiplied every lakh nearly 14 times.
Timing the markets could be suicidal as valuations and volatility put the markets in a see-saw mode.
As an individual investor, you need to sit tight over high conviction stocks and invest consistently to see the magic of compounding.
Because 2022 could be extremely profitable, over time, provided you reset your portfolio with the right kind of safe assets and safe stocks.
Moving on to stock specific news...
Sun Pharma is among the top buzzing stocks today.
Sun Pharma on Tuesday said its subsidiary has received emergency use authorization (EUA) from the Drugs Controller General of India (DCGI) to manufacture and market a generic version of MSD and Ridgeback's antiviral drug molnupiravir under the brand name Molxvir in India.
The DCGI has approved molnupiravir for the treatment of adult patients with Covid-19 and who have a high risk of progression of the disease including hospitalization or death.
Shares of Sun Pharma are presently trading up by 1.7%.
Note that apart from Sun Pharma, several other pharma companies including Cipla, Hetero and Torrent Pharma also announced plans to market their versions of antiviral drug Molnupiravir.
All these companies have received approvals from the DCGI to manufacture and market their versions.
Earlier this year, these companies had signed non-exclusive voluntary licensing agreements with MSD to manufacture and supply the generic version of molnupiravir in over 100 low and middle-income countries (LMICs), including India.
The DCGI, based on the review of clinical data of Molnupiravir approved it for the treatment of adult patients with Covid-19.
Pharma stocks are trading on a mixed note today with Sun Pharma and Strides Pharma leading gains.
Disclosure: Equitymaster Agora Research Private Limited (Research Analyst) bearing Registration No. INH000000537 (hereinafter referred as 'Equitymaster') is an independent equity research ...
more


