Sensex Today Ends 126 Points Lower; Infosys & SBI Top Losers
After opening the day on a negative note, Indian share markets continued the downtrend as the session progressed and ended the day lower.
Benchmark indices fell on Friday dragged by a slide in information technology (IT) and bank stocks, while inflation concerns in the United States also weighed on investor sentiment.
At the closing bell, the BSE Sensex stood higher by 126 points (down 0.2%).
Meanwhile, the NSE Nifty closed lower by 60 points (down 0.3%).
Tata Motors and Nestle were among the top gainers today.
Infosys and SBI were among the top losers today.
Check out the NSE Nifty heatmap to get the complete list of gainers and losers.
The Gift Nifty was trading at 19,728, up by 53 points, at the time of writing.
Broader markets ended on a positive note. The BSE Midcap index and the BSE SmallCap index ended marginally lower.
Sectoral indices ended mixed with stocks in the metal sector and banking sector witnessing buying. Meanwhile, stocks in the auto sector and realty sector witnessed selling pressure.
Shares of Nestle and Bosch hit their 52-week highs today.
Now track the biggest movers of the stock market using the stocks to watch today section. This should help you keep updated with the latest developments...
Asian share markets ended on a negative note. The Hang Seng fell 2.3% while Nikkei ended 0.6% lower. Meanwhile Shanghai Composite closed 0.% lower.
The rupee is trading at 83.25 against the US$.
Gold prices for the latest contract on MCX are trading up by 0.6% at Rs 58,311 per 10 grams.
Meanwhile, silver prices for the latest contract on MCX are trading 1% higher at Rs 69,81 per kg.
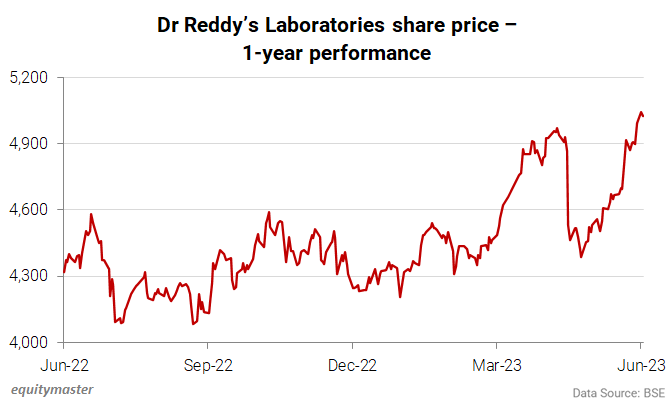
Why Dr Reddy's Share Price is Falling
In news from the pharma sector, Dr Reddy's Laboratories stock price declined 2.3% in trade in October amid a weak domestic market. The US Food and Drug Administration (USFDA) completed an inspection at the pharma major's manufacturing facility and issued a Form 483 with nine observations.
The USFDA completed a product-specific pre-approval inspection (PAI) at the pharma player's biologics manufacturing facility. The inspection was conducted from 4 October to 12 October at the firm's facility in Bachupally, Hyderabad.
A Form 483 is issued by the USFDA to notify the company's management of objectionable conditions.
It is issued towards the end of an inspection when the inspector has observed any conditions that may be seen as violations.
From June 2022 to June 2023, it has rallied around 14.8%.
SpiceJet soars 10% today. Here's why.
Moving on to news from the airline sector, SpiceJet sector, shares of low-cost carrier SpiceJet surged on October 13 ahead of a hearing in the Delhi High Court in its case against Sun Group chairman Kalanithi Maran. The case pertains to an arbitral award in favor of Maran.
In February 2015, Maran and KAL Airways, his investment vehicle, transferred their 58.5% in SpiceJet to Singh, who took on the airline's liabilities of around Rs 15 bn. As part of the agreement, Maran and KAL Airways said they paid SpiceJet Rs 6.8 bn for issuing warrants and preference shares. Maran alleged that the warrants and preference shares were not allotted and initiated arbitration proceedings against SpiceJet and Singh.
In July 2018, an arbitration panel rejected Maran's claim of damages of Rs 13.2 bn for not issuing warrants to him and KAL Airways but awarded him a refund of Rs 5.8 bn plus interest. SpiceJet was permitted to furnish a bank guarantee for Rs 3.3 bn and make a cash deposit of the remaining sum of Rs 2.5 bn.
It added that it acknowledges the legal process and is committed to complying with all court directives and obligations in the Credit Suisse matter and will make the payment of US$ 1.5 m as per the court directive.
The company's shares are down by more than 25% in 2023.
Lupin receives USFDA approval
Moving on to news from the pharma sector, Lupin Limited, a global pharmaceutical leader, announced on Thursday that it has secured tentative approval from the United States Food and Drug Administration (USFDA).
This approval pertains to its Abbreviated New Drug Application for an Oral Solution containing Calcium, Magnesium, Potassium, and Sodium Oxybates at a concentration of 0.5 g/mL.
This medication aims to introduce a generic equivalent of Xywav® Oral Solution, also at a concentration of 0.5 g/mL, which was originally produced by Jazz Pharmaceuticals Ireland.
This product will be manufactured at Lupin's Somerset facility in the US.
Lupin is exclusive first-to-file and may be eligible to receive a 180-day exclusivity period at product launch.
The net product sales for Calcium, Magnesium, Potassium, and Sodium Oxybates Oral Solution (RLD Xywav®) were USD 958.4 million for the year ending December 2022 and USD 604.3 million for the first six months of 2023.
Lupin is a top pharma company in India that has solid growth in sales and profits and a high Return on Equity (ROE).
More By This Author:
Sensex Today Falls 300 Points, Nifty Near 19,700Sensex Today Ends 65 Points Lower; Laxmi Organic Falls 4%
Sensex Today Trades Lower; TCS & Nestle Top Losers
Disclosure: Equitymaster Agora Research Private Limited (Research Analyst) bearing Registration No. INH000000537 (hereinafter referred as 'Equitymaster') is an independent equity ...
more


