3 Promising Gene Therapy Stocks

- Biotech stocks and the broader healthcare sector remain under pressure.
- Promising opportunities exist in the small-cap and mid-cap segments.
- Gene therapy remains the emerging scientific frontier in biotech.
- 3 promising small-cap gene therapy stocks for a biotech portfolio.
Biotech Pulse
The Nasdaq Biotech (IBB) and the S&P Biotech (XBI) indexes have struggled since March as the broader healthcare sector (XLV) declined following growing momentum amongst the presidential candidates for a universal healthcare plan. This was at a time when the broader market indexes, the Nasdaq Composite (QQQ), and the S&P 500 (SPY), set new highs, prior to the recent pullback.
The trade friction has a more restrained immediate impact on the biotechs and affects the broader biopharma sector mostly in the area of patent protection and market exclusivity terms that are negotiated. But the flight from risk, as expectations of an imminent trade resolution were derailed, hurt the speculative sector, like the biotechs, more sharply.
The healthcare overhaul and uncertainty will remain an ongoing theme until the elections, leading to higher sector volatility and restrained gains, as highlighted in the article 2019 Outlook: Biotech Bonanza: 2019 Outlook On An Uphill Battle. Nonetheless, there will be attractive opportunities, particularly in midcap and smallcap segments, to outperform the biotech indexes. One segment of biotechs that is attractive for its emerging potential is discussed below.
Gene Therapy
Source: BioSpace.com
Gene therapy is a generational scientific advancement with the potential to make a growing and gigantic impact on healthcare.
Genes are part of the DNA that provides the blueprint instructions to the cells for making proteins. A mutation or a defect in the gene can prevent the cells from creating an accurate expression of the protein or a diminished ability to do so. Gene therapy is the science of altering DNA to restore normal protein expression by the cells or even silence proteins that may cause debilitating diseases.
There has been a surge of interest in gene therapy as key milestones are being achieved, particularly in rare diseases, and the scientific advances are pushing the frontiers of genomic medicine. Significant progress has been made in improving the understanding of using different delivery vehicles or vectors to safely deliver the therapeutic DNA altering payload. CRISPR, which makes addition and deletion edits directly to the DNA strand, is a popular editing tool in gene therapy. Another approach to correcting gene mutation that is gaining popularity is the use of viral vectors to deliver the therapeutic payload. Any viral vector needs to be non-pathogenic in order to minimize any immune response so that the vector particle, and the embedded payload, is not destroyed by the antibodies.
Two types of viral vector platforms being used are the Adeno-Associated Virus (AAV) for the in vivo gene therapy and the Lentivirus from stem cells for ex vivo gene therapy. Using particles of AAV, which is not known to cause disease and typically has a mild immune response, is garnering a lot of interest as a virus vector in gene therapy for many targeted diseases. But challenges exist particularly around AAV-antibodies which can pre-exist in patients. Research has advanced to a point where variants of AAVs are being used to improve the immunogenicity profile, which means minimal immune reaction when a viral vector is introduced in the body.
There are a growing number of gene therapy companies with a wide range of market caps. A few of them include Editas (EDIT), Crispr (CRSP), Solid Biosciences (SLDB), Spark Therapeutics (ONCE), Homology Medicines (FIXX), Sarepta Therapeutics (SRPT), Bluebird (BLUE), Regenxbio (RGNX), PTC Therapeutics (PTCT), Meiragtx (MGTX), and Intella Therapeutics (NTLA).
Investor interest in gene therapy companies has also been spurred by acquisition activity at sizable premiums – Spark Therapeutics was acquired by Roche (RHHBY) earlier this year for an over 100% premium, and Avexis was acquired by Novartis (NVS) early in 2018 at an over 70% premium.
Here is a look at 3 promising small cap gene therapy companies.
Uniqure (QURE)
The company is a platform driven gene therapeutic company. A robust technology platform allows multiple shots at the goal and thus reduces a risk profile compared to single-product biotech.
Uniqure has a modular gene therapy platform, which allows it to leverage the core technology for a variety of targeted therapies using its AAV vector delivery system. The Company uses an engineered virus variant called AAV5, which its research has shown to have a favorable immunogenicity profile even in the patient population with pre-existing AAV neutralizing antibodies. Uniqure has exclusive worldwide rights to the AAV 5 variant used in therapies delivered to the brain or liver and uses its own manufacturing lab and a proprietary process to produce its AAV-based gene therapies.
Uniqure is working on a few liver and neurological targets. It’s most advanced therapeutic programs are in hemophilia, a blood clotting gene disorder, while other programs are in preclinicals or Phase I.
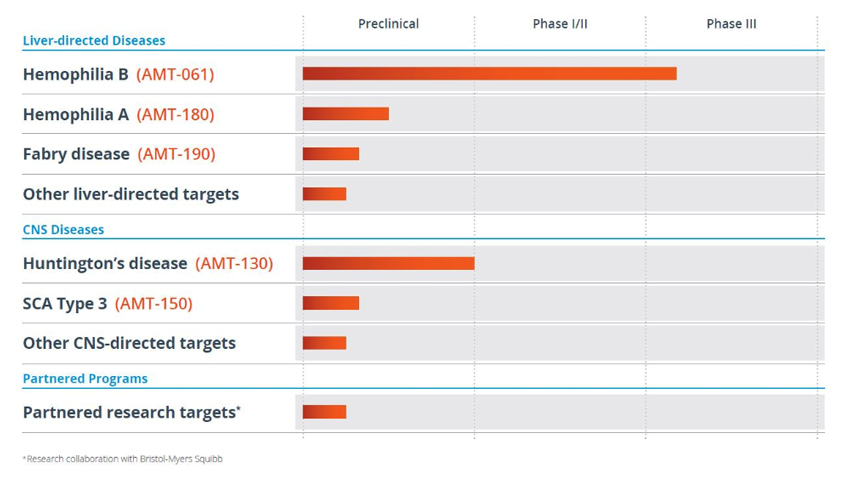
Uniqure Product PipelineSource: Uniqure (click for a clearer image)
Last week, the Company announced further data on its promising and clinically most advanced program, AMT-061, for Hemophilia B and the results were strikingly promising. The Hemophilia B disorder is caused by a missing or defective copy of Factor IX or FIX, a blood clotting protein, and is both hereditary as well as a spontaneous mutation with no family history. In the Phase 1b study of AMT-061, an investigational AAV5-based gene therapy for the treatment of patients with severe and moderately severe hemophilia B, all the 3 patients administered showed sustained FIX levels of the protein and two of the patients recording readings within 50% of normal range at the 6-month interval after dosing. Very important, there were no safety issues with regards to tolerability and no patient required immunosuppression relating to the therapy, this despite all 3 patients showings low levels of pre-existing neutralizing AAV5 antibodies. The sustainability of the improvement after 6 months and the safety profile with no adverse immunological reaction was striking.
Last year, Uniqure began its ‘HOPE-B’ Phase 3 trial of AMT-061 for adults with moderately severe or severe hemophilia. The trial will evaluate the efficacy and safety of AMT-061. The enrolled patients will be first observed for six-months on their current standard of care, and thereafter receive a single dosage of AMT-061. The primary outcome measure is the assessment of Factor IX activity 26 weeks after the dosing. The trial is expected to be fully enrolled, with about 50 patients, later in 2019. Earlier, in February 2019, the Company announced the treatment of the first patient in the trial. The 6-month period after dosing should end around August 2019, and one would anticipate the company providing some information thereafter. Investor focus will remain on the results of the AMT-061 drug Hemophilia B and whether the program can advance towards commercialization.
As of the end of the March quarter, the company had $210 million in cash and equivalents and expects this liquidity to fund operations into 2021.
Voyager Therapeutics (VYGR)
Voyager is a clinical-stage gene therapy company focused on neurological diseases. The Company uses AAV vectors in developing its gene therapies and has the production ability to engineer these vectors at scale with the appropriate therapeutic payloads. Voyage Therapeutics has a core AAV-driven platform to develop vectorized antibodies for various diseases.
The Company’s product pipeline is targeting some of the hardest neurological diseases to resolve, which also include Parkinson’s disease, Amyotrophic Lateral Sclerosis (ALS), Huntington’s disease, Alzheimer’s disease, and other diseases related to defective or excess aggregation of tau proteins in the brain.
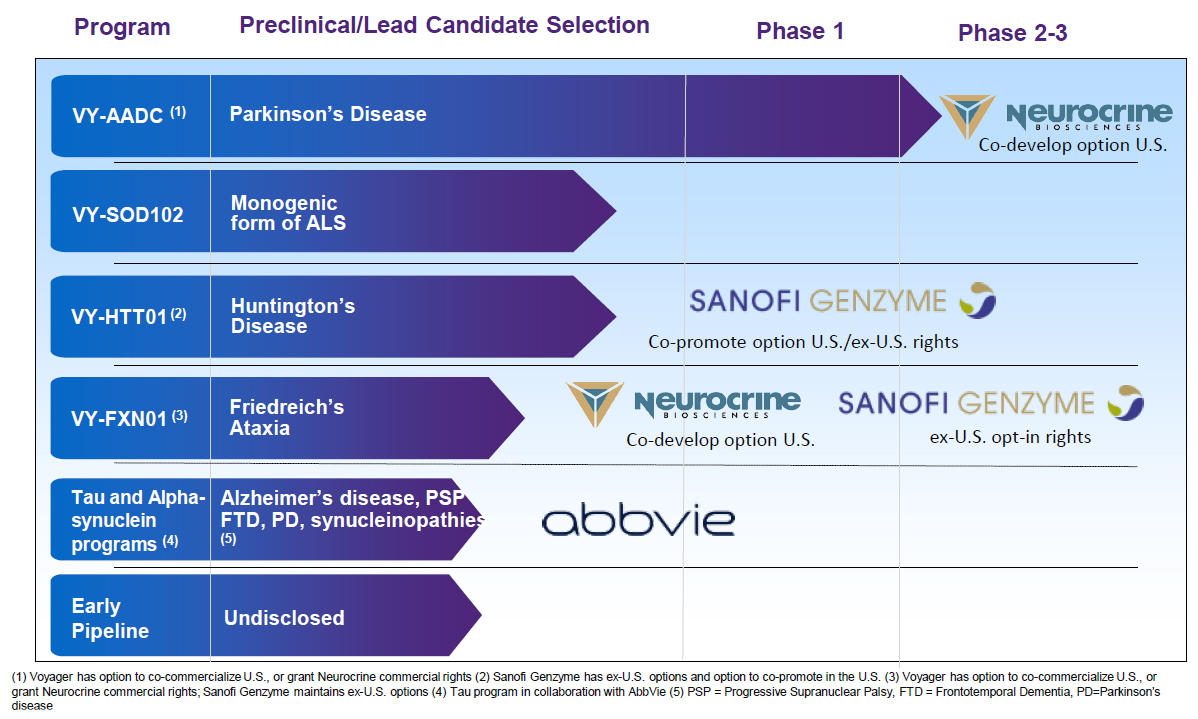
Voyager Therapeutics Product PipelineSource: Voyager Therapeutics (click for a clearer image)
In March 2019, the Company began a collaboration with Neurocrine Biosciences (NBIX) for gene therapy drug development targeting Parkinson and Ataxia. The announcement of Phase 1b interim trial data in March from the administration of VY-AADC, demonstrated safety, tolerability, and improvement in clinical measures. The other programs are in preclinical stages and have garnered significant collaboration interest. The company is collaborating with Abbvie (ABBV) on Alzheimer and other neurodegenerative diseases, and with Sanofi-Genzyme (SNY) on Huntington Disease and Ataxia. There are a variety of upfront and milestone payments associated with the collaborations.
Investor focus will remain on continued progress in its various programs, including long-term data from the Phase 1b trial for VY-AADC. Voyager has also initiated a Phase 2 trial referred to as RESTORE 1, for patients with Parkinson’s disease for at least 4 years. The primary endpoints will be measured after 12 months. The trial is expected to fully enroll by mid-2020.
The Company had a cash position of ~$360 million at the end of the March 2019 quarter and expects to have sufficient liquidity to fund operations until mid-2022.
Audentes Therapeutics (BOLD)
Audentes is a gene therapy company leveraging its AAV-based vector technology platform to focus on rare neuromuscular diseases. The company has its own manufacturing expertise to engineer the proprietary AAV vectors to deliver the therapeutic payloads.
The Company’s product pipeline is targeting Myopathy, Pompe, and Duchenne Muscular Dystrophy (DMD).
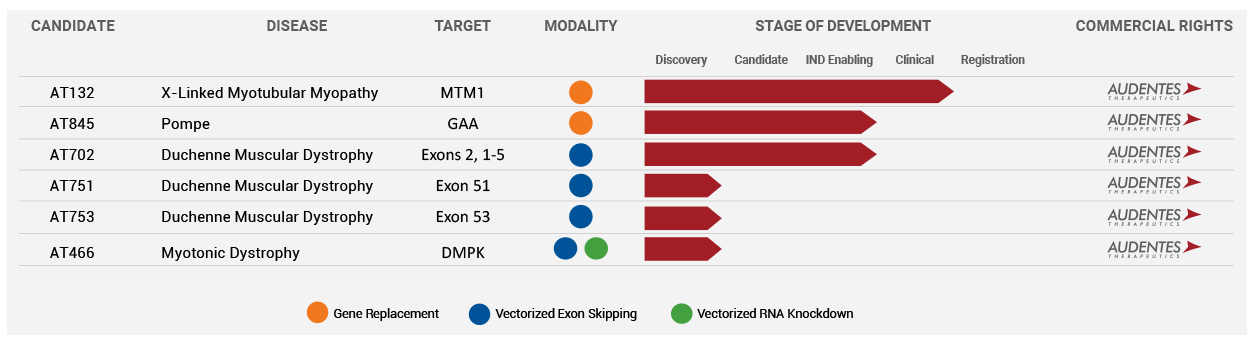
The most progressed program is for the candidate AT132 in Phase 1/2 to treat X-linked Myotubular Myopathy (XLMTM). This is a life-threatening disease characterized by extreme muscle weakness and a high mortality rate of 75% by age 10 for those who survive past infancy. It is caused by mutations in the MTM1 gene that leads to dysfunctional production of myotubularin protein. The AT132, which is a novel AAV8 vector with a functional copy of the MTM1 gene, has received a Fast Track and Orphan designation from the FDA. The ASPIRO Phase 1/2 study is evaluating the safety and efficacy of AT132.
Earlier in May, new positive data was released from the ASPIRO study demonstrating a good tolerability and safety profile, along with a sustained reduction in ventilator dependence for the 6-patient cohort. The company is working on gene therapies for some complex neuromuscular diseases and the progress of its products for Myopathy (AT132), Pompe (AT845), and Duchenne Muscular Dystrophy (AT702) will be watched carefully by investors. On AT132, Audentes expects to select an optimal dose during this quarter, with the next clinical data presentation in October. In the case of AT845 for Pompe disease, the Company expects to file an IND in the third quarter of 2019, and for DMD, an IND filing is expected in 2020.
The Company had a cash position of $375 million at the end of the March 2019 quarter and expects this liquidity to provide sufficient runway into 2021.
Source: iphoto
Conclusion
Gene therapy stocks are pushing a new frontier of science. Interest has continued to rise due to the enticing promising of potentially curing a spectrum of diseases and conditions in a more targeted, effective, and durable manner. New ideas in gene therapy are finding ready and eager financing and the ambitions are growing. The most recent announcement was the launch of a gene therapy effort targeting cardiovascular health,by start-up Verve Therapeutics and backed by Google (GOOG) parent Alphabet.
However, as investors realize, any generational advance, similar to a paradigm change, in tackling diseases is not going to be without major setbacks. Such has been the case in gene therapy as well. It is better to pursue a portfolio approach in this dynamic segment and the above three companies, Uniqure, Voyager and Audentes, are some of the speculative promising prospects in gene therapy. These companies have a gene therapy development platform, are targeting multiple indications, and have achieved some important initial successes. And they are just a few of a growing pool of gene therapy companies targeting complex diseases with their high potential and innovative gene therapies.
Some other promising biotech companies, which may be now or in the past be part of the Prudent Biotech model portfolio or the Graycell Small Cap portfolio, include Biohaven Pharmaceutical (BHVN), Sage Therapeutics (SAGE), Amarin (AMRN), GW Pharmaceuticals (GWPH), Denali Therapeutics (DNLI), Acadia Pharmaceuticals (ACAD), Arena Pharmaceuticals (ARNA), Array BioPharma (ARRY), Ascendis Pharma (ASND), Pacira Pharmaceuticals (PCRX), China Biologics (CBPO), Uniqure (QURE), Moderna (MRNA), PTC Therapeutics (PTCT), Mirati Therapeutics (MRTX), Ra Pharmaceuticals (RARX), Bluebird Bio (BLUE), Allogene Therapeutics (ALLO), Portola Pharmaceuticals (PTLA), Crispr Therapeutics (CRSP), Global Blood Therapeutics (GBT), Axsome Therapeutics (AXSM), TG Therapeutics (TGTX), Medicine Company (MDCO), Ziopharm Oncology (ZIOP), Coherus Biosciences (CHRS), and Arrowhead Pharmaceuticals (ARWR). This is just a short list.
As always, use a portfolio approach to invest, particularly with biotech stocks, to overcome the inevitable errors.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.




You left out the big dog, $RGNX