Market Briefing For Tuesday, Sept. 23
Market 'anesthesia' - was expected to relieve the pain inflicted by super-cap selling, at least temporarily on Tuesday and perhaps even Wednesday, and that's what we've got going. Too many are trying to rationalize whether these stocks are investments or trading buys 'yet' and the answer is slightly variable, but generally not so investment wise, although for a trade that's exactly what we projected for an intraweek bounce.
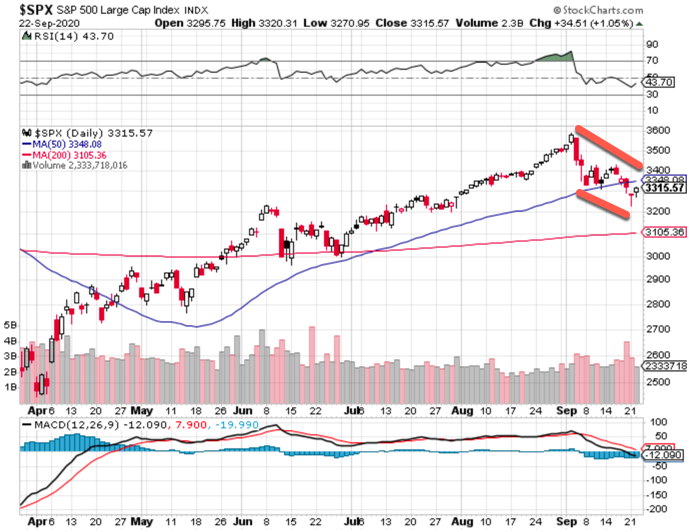
This entire move is technically based, not particularly influenced by a Fed Chairman's and Treasury Secretary's remarks at Congress, nor by the vacillations by the CDC as to the flip-flopping with regard to the precautionary warnings to avoid COVID exposure. (Hint: the weekend 'erroneous draft version' was likely accurate and withdrawn so they can edit and tone it down before posting it again perhaps later this week. Basically its fear-inducing and CDC probably caught flack yet again from The White House on it.)
Executive summary:
- Air Force 2 made an emergency landing in New Hampshire, with Vice President Pence aboard, after a 'bird strike', no injuries, not reported yet whether the plane remain on the ground for repairs or can resume its return to Andrews AFB.
- Tuesday's dip and rebound was facilitated by the Fed's Evan's remarks saying the Fed might 'hike' rates before we got to 2% inflation, although that will likely be viewed as misunderstood and countered by other Fed officials later in the week.
- Personally I'd be fine with rates firming sooner rather than later, because it would reflect better business conditions and not have the Fed as far 'behind the curve' as they are likely to find themselves down-the-road a bit.
- The U.S. House passed a bill late tonight to avoid a Government shutdown, this was widely expected to be passed to avoid any such problems.
- S&P action is almost entirely technical in nature, with no particular departure from our weekend forecast for a breakdown after Quarterly Expiration, a rebound that's generally back to the breakdown point, and then potentially another phase lower.
- During this activity certain stocks are in better position, including some 'COVID-19' plays outside of the big-pharmas.
- Some of this focus relates the FDA emphasizing tightening of restrictions on any post-Phase 3 approvals (safety, efficacy and basically lots of test subjects), really wanting to be sure they work and are safe before wide deployment.
- This is not surprising (outside of some limited releases to first-responders, maybe medical workers, or some in the high-risk elderly/immune-compromised realm.
- Non-vaccine treatments (therapeutic drugs) are more important than vaccines in the shorter-term, and those are not 'yet' available, but may be proven faster than vaccines, especially where infused into symptomatic patient.
- That's because if a patient is 'responsive' it will be known within a week or two.
- Some smaller pharmas are willing to do their Phase 1 only in early symptomatic patients (where it's harder to determine if a neutralizing antibody works or patients just recover as most do anyway within a couple weeks), Lilly is one such pharma (LLY).
- Others are willing to do Phase 1 on 'hospitalized' patients, where outcomes really are clearly definitive, and success should be evident within days (hence leaving a possibility for 'trials' to be halted early and moved to wider use if that's the case), and of course here we're referring to Sorrento, which has approval to go forward with Phase 1 of their STI-1499 in both the United States and presumably Brazil.
- Meanwhile the reticence to approve vaccines might actually help interventions of a novel type, like Heat Biologics (HTBX) T-Cell simulator, they expect another patent to be be issued tomorrow, although that is broadly anticipated.
- Today Sorrento (SRNE) rallied smartly again, this time on really Positive results from their non-Opioid pain reliever Phase 1b drug Trial, that was so impressive it seems they go directly into Phase 3, with favorable comments from the lead Trial Direction (at Harvard / Brigham's & Women's) express very favorable views.
- The significance of this relates to analyst optimism on this RTX epidural drug well before COVID was discovered, hence last November's price target of 21 by a firm based just on the 'pain relief' drug, with anything else to fight cancer a bonus (so now part of the most optimistic views have to include possibly a COVID drug too, not to mention the tests.. but the irony is the non-Opioid alone is meaningful).
- Sorrento's RTX likely will target multi-millions of patients, that's how big markets are for non-opioid pain therapy, clinical trials show rapid onset relief (day 1) and extended pain relief out to 84 days, so COVID and cancer aside, it's a big deal, so of course we'll be watching the Phase III trial and that alone can zoom the stock if successful, it along the line something succeeds with COVID.. well ..higher yet.
- There's no change in our market view, and for now we suspect higher prices but for S&P it should be tempered since many will realize this should be a rebound, as opposed to any sort of turn from a washout low that might be in the future.
Speaking of pain relief . . . as I mentioned, one analyst who gave Sorrento a price target of 21 in November of 2019, before the COVID pandemic, based his price goal on RTX (the drug trial progress reported just today) 'alone'. He made the point that other cancer therapies would be additional potential if or as progress occurs.
While we have no idea what that analyst thinks now, any COVID drugs would enhance SRNE prospects even more. Of course there are risks with that stock and shorts after it all of the time, perhaps even litigation between the Company and bears (who might be so adamant at the behest of a billionaire bear likely near being compelled to settle with the firm for a prior issue of buying then killing a possible cancer treatment drug.. I have no idea if that's where the source of acrimony stems from, but might be.. in any case the shorts get freaked but at various points it helps, as some frantically cover).

Now let me mention more about RTX (the abbreviated term for the pain relief epidural drug). Besides cancer, where about a 30% reduction in pain was seen, it's also being trialed on arthritis patients. That's a huge market too. Especially to use an example, think about how many knee replacement surgeries occur annually. If RTX reduces the pain sufficiently, many patients would be content and thus prefer to avoid surgery.

Further, once / if FDA approves for pain, I can envision RTX injections being required by insurance companies before knee-replacement surgery is authorized, because if (and appears it does) pain relief was sufficient they'd want patients to do that as an epidural before resorting to any surgery. Big cost savings. Think about it. Non-Opioid pain relief is a very big deal, and is a good reminder of Sorrento's overall portfolio.

Non-Opioid pain relief alone validates this company, and today's market action tends to concur (up 15% on the news). Read comments by the lead Trial Director for a fuller appreciation of RTX potential. The Harvard Medical / Brigham & Women's doctor who did the research and supervised the trial is an anesthesiology professor and is quoted in the Company's presentation made today. The active (pending Phase 3) trial is being conducted at Brigham & Womens, as well as Duke University, and Sylvester Cancer Institute at the University of Miami (also Herman Drive Surgical in Houston).

In-sum: the overall market evolution continues on course, Wednesday initially could be slightly up and then encounter some resistance for the S&P, but possibly be able to hold traction overall for the moment. Perhaps an up-dip-up session, though it's not terribly consequential at the moment (barring drama or exogenous events).



