Consider The 2-3x Upside In This Most Overlooked Hepatitis B Stock
GlobeImmune (GBIM) IPO’d in June to little fanfare and has underperformed the biotech sector considerably since. Now, however, this overlooked stock is trading at all-time lows and only marginally above its cash position, with a key driving event in the next six months that should put the company back on the radar, particularly for investors focused on the evolving landscape for infectious diseases affecting the liver.
GlobeImmune develops targeted treatments for oncology and infectious diseases based on the company’s proprietary Tarmogen platform. Essentially therapeutic vaccines, Tarmogens are designed to activate the immune system to target particular proteins on infected or malignant cells. The technology has been around for some time - GBIM was founded as Ceres Pharmaceuticals in 1995 - and investors have watched therapeutic vaccines fail in the recent past. These have contributed to little interest in GBIM.
Despite little to show in nearly 20 years of drug development, the company has made some interesting advancements in the last five years, one of which informs exclusively our interest in trading this illiquid stock. GlobeImmune’s lead infectious disease candidate, GS-4774, is partnered with Gilead Sciences (GILD) for the treatment of hepatitis B. Two phase 2 proof of concept studies are ongoing and one will readout in the first half of 2015, testing GS-4774 combined with standard of care HBV regimens in treatment-experienced chronic HBV patients. Gilead recently initiated a third trial with GS-4774, following previously treated patients once they have completed participation in another -4774 trial.
Further, GlobeImmune is partnered with Celgene (CELG) for one of its early oncology candidates, also a Tarmogen-driven therapeutic. GBIM has been under pressure since mid-October when the company released data from a phase 1 study of GI-6301, a Celgene-partnered candidate, in the rare bone cancer, chordoma. For traders/investors interested in the forthcoming HBV data, this makes for an attractive opportunity to get involved in the stock at all-time lows.
- GBIM is partnered with two leading biopharma companies, Gilead and Celgene, on its proprietary Tarmogen platform. Stock has underperformed since June IPO and is overlooked as an early HBV story.
- Risk/reward coming into year-end is incredibly favorable: the stock has cash support at roughly 50% of the current valuation, and we see the stock returning to $10 (IPO price) prior to next major inflection points, early next year. We see GBIM as a table-pounder into year-end.
- Own GBIM at all-time lows (around $5.50) for short-term trade as investors come around on beat-down story (avoid holding through data), OR long-term and riskier holding through upcoming HBV readouts. Early entrance may allow for "free" involvement (playing with profits) when HBV data emerges.
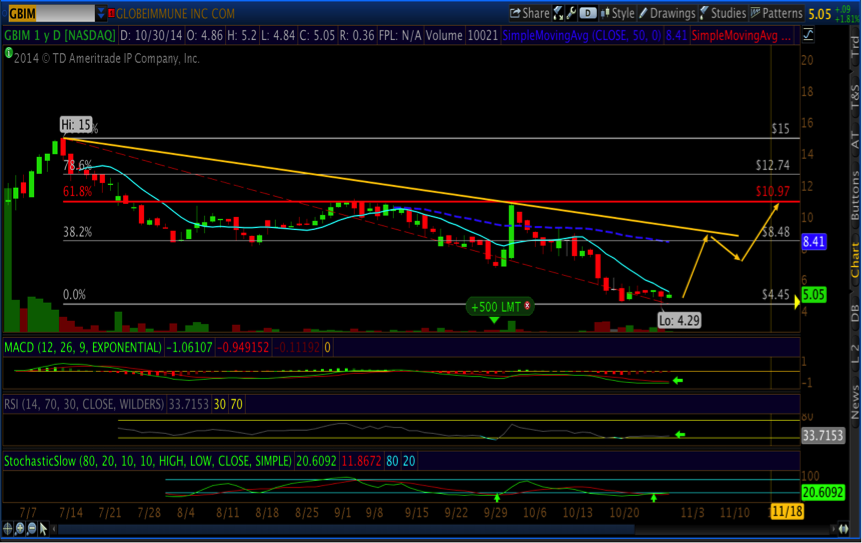
After the sharp sell-off in GBIM shares on thin volume (<500,000 shares) with the release of initial chordoma data in mid-October (details below), risks in GBIM are limited setting up an attractive risk-reward ahead of value unlocking data in 1H2015. Importantly, all oscillators are in very oversold conditions, and we believe initiating positions ahead of AASLD (November 7th) and pivotal data in 1H2015 could see shares test $11.00 (61.8% retracement) that implies only a 5% probability of success. As a trade, this allows investors to go into the HBV event risk-free, with only profits at risk.
Leading into the event, we’re defining “good data” as a 30-50% improvement in Viread monotherapy response rates (11%), or reduced time to response, which may propel shares to our fundamental base case DCF valuation for GS-4774 of $17/share. Any beat of our expectations and shares could trade to a 50% probability adjusted approval DCF valuation of $36 per share.
In the near-term we think shares test the down trend at least once, currently at $8.48 (66% upside), and upon favorable data in 2015 shares could easily triple past all time highs of $15 per share. On Friday, shares closed up 11% at $5.63, generating a mechanical buy signal on the Slow Stochastic, with an imminent MACD cross, albeit still below the zero line. In short, we think we see $8 before we see $3.
GlobeImmune, Inc., Background
Established as Ceres Pharmaceuticals in February of 1995 to develop Targeted Molecular Immunogens – Tarmogens – based on research in-licensed from the University of Colorado, the company changed its name to GlobeImmune in 2001 and went public this June with a $17.25M initial offering. The IPO follows a 2012 attempt to come to market, which the company pulled in October of 2013. Clearly, the Street takes a dim view of GBIM and/or the Tarmogen therapeutic approach, considering the company’s failure to list publicly two years ago as the biotech IPO window was wide open. This, and the company’s long development track record, is certainly worth acknowledging as “hair” to the story.
What is a Tarmogen, how does it work in HBV?
GS-4774 (Tarmogen from GlobeImmune) is derived from a heat-inactivated yeast vector containing the entire HBV antigen signature (core, surface, and envelope) being developed as a therapeutic vaccine. The proposed mechanism of action is believed to be re-sensitization of dendritic cells to initiate a targeted T cell immune response. Additionally, the GS-4774 Tarmogen expresses a fusion protein utilizing a highly conserved sequence of the hepatitis B virus that is homologous in all four major HBV genotypes and is thought to be pangenotypic. We note that ARC-520, Arrowhead Research’s (ARWR) highly anticipated HBV candidate, consisted of two siRNA’s with the hopes to have broader genotype coverage, but it validates the importance of GlobeImmune’s pangenotypic approach.
HBV is characterized by its capacity to inactivate the immune system, or deplete the immune system’s capacity to elicit an immune response to the HBsAg (immune exhaustion), and actively inhibits dendritic cells. This is where Tarmogens may prove unique, as they are absorbed and processed by dendritic cells into peptide fragments that are then presented onto the exterior with class II MHC complexes. A brief analogy to small molecules: think of Tarmogens as the pro-drug, and the dendritic cells (liver) as the bioactivation site for releasing an active form. In this case, dendritic cells having processed Tarmogens containing the HBV antigens are cleaved into peptide fragments that are then transported to the cell surface in conjunction with cytokine release in a traditional autocrine response.
These antigen-presenting cells now display on class II MHC-Tarmogen Peptide complexes on their extracellular membranes that rely upon IL-12 to activate CD-4+ T-helper cells (Th). Once IL-12 is released, it chemo-sensitizes circulating CD-4+Th cells toward the antigen-presenting dendritic cells (chemo-sensitization) that are now able to communicate with the dormant immune system through a traditional cell-cell, receptor-ligand interaction between the class II MHC-Tarmogen Peptide antigen presenting cells and the CD-4 Th cells.
The ultimate immuno-pharmacological objective of this cascade is to stimulate the release of Interferon-gamma (IFNg), that in this context re-activates dormant CD-8+ Killer T- cells, becoming immunocompetent to target diseased cells with the same antigen and to induce cytotoxicity through a variety of mechanisms. Systemic interferon therapy results in a similar pharmacologic effect; however, we adamantly caution investors not to oversimplify this complex pathway by deducing “Tarmogens are similar to interferon.
While acknowledging the fact that this program has significant early stage risk, on an unproven platform, the mechanistic rationale remains sound. We heavily discount projected cash flows at 20% WACC, and risk-adjust by 25% below the average probability of success (30-35%) for a Phase 2 asset. Lastly, in our GBIM model we only value the HBV program despite two other Phase 2 assets in oncology with one already licensed to CELG, further validating the platform. Celgene has an option to opt-in on the remaining oncology asset, triggering a milestone payment as soon as year-end.
GBIM has 3 other programs in preclinical development and according to management at least one will be held for proprietary development with an affinity toward tuberculosis (TB), a program GBIM has received $4M in grant money from thus far.
For modeling purposes (pricing and market share), we utilize several layers of conservatism: we examined existing sell-side HBV models, including ALNY-HBV, GILD’s Viread/HepSera/pipeline, and Arrowhead’s ARC-520. We assume no increase in treatment rates above current levels and assume only 1% growth per year – both extremely conservative.
Valuation:
Sales Model

DCF Model

DCF Input Summary and Valuation


Gilead Partnership and GS-4774 are the Current Reasons to Own
In October of 2011, global antiviral leader Gilead Sciences licensed from GlobeImmune exclusive worldwide rights to the company’s Tarmogen products directed at HBV. Gilead paid $10 million upfront and agreed to fund a phase 1a safety and dose-ranging study of GS-4774 in healthy patients. Under the licensing agreement, GBIM is eligible for $130 million in development and regulatory milestones; tiered royalties in the upper single digits to mid-teens on any commercial sales of GS-4774; and up to $40 million in sales milestone payments.
The company has since received $5 million in milestone payments associated with the Phase 1a, and two new Phase 2 clinical trials are underway, for which Gilead is solely responsible.
The phase 1a study, GI-13020-01, was a randomized, open-label, dose-escalation trial completed in August 2013 assessing the safety, tolerability, and immunogenicity of GS-4774 in 60 healthy adults. Three doses were evaluated: 10 YU (yeast unit, =10 million yeast cells), 40YU and 80YU, each in 20 subjects. Two dosing regimens (weekly & monthly) were evaluated in each cohort. Though measurement of surface antigen reduction was not included in the phase 1 trial, an immune response was observed in 88% of subjects, higher at the highest dose, by at least one T-cell assay. IFN-γ-producing T-cells specific to HBV antigens were detectable in 51% of subjects. ELISpot response was observed at all doses, with the highest frequency of responders at the highest dose (80YU, 74%). Proliferative responses to HBV recombinant antigens were observed in 90% of subjects. This confirms and validates similar pharmacodynamics properties observed with preclinical studies that can be found here.
Following the phase 1 results, Gilead initiated two 175-patient phase 2 trials of GS-4774 in combination with standard antiviral regimens: one in treatment-experienced HBV patients and one in treatment-naïve. The primary endpoint for both trials is mean change in log10 IU/mL serum hepatitis B surface antigen (HBsAg) from Baseline to Week 24. The first (NCT01943799) began in September of 2013 and was fully enrolled around mid-year; 48-week results are expected in the first half of 2015. Gilead initiated the second trial (NCT02174276) in July of 2014.
GlobeImmune guides for treatment-experienced results in the first half of 2015, though this is not entirely clear why. The primary endpoint in this, and the second study, is HBsAG reduction at week 24, and the trial lists a primary completion date of September 2014. Presumably, GILD does or will soon have this data in-hand. After discussion with management, we assume guidance from Gilead is for topline results once 48-week data are available as well, hence guidance for 1H15 data release.
Importantly, Gilead has initiated a third clinical trial/patient registry with GS-4774, listed on clinicaltrials.gov just this October. The study is designed to “evaluate the long term effects of hepatitis B virus (HBV) treatment on the HBV serologic changes and HBV DNA levels through Week 144,” specifically in patients who received GS-4774 in a previous trial we view any increased rate of clearing surface antigen relative to Viread as a major positive. The study start date is November 2014, with a primary completion date of June, 2018. Interestingly, the trial’s primary endpoint is the proportion of participants with HBsAg decline ≥ 0.5 log10 IU/ml from baseline through Week 48. This initiation date coincides roughly with completion of the 24-week primary endpoint in the first, treatment-experienced GS-4774 study, which likely informed the decision to follow patients. Bare in mind, the two phase 2 trials are open-label: GILD has access to data as it becomes available.
While we caution investors against inferring too much from the initiation of this patient registry, this is incremental validation that, in the least, GS-4774-treatment is worth following for outcomes.
On HBV and Current, Lackluster Treatment
Guidelines state that patients should be considered for antiviral treatment only when serum HBV DNA is above 2,000 IU/ml (that is, approximately 10,000 copies/ml), and/or serum ALT levels are above the upper limit of normal (ULN), and liver biopsy shows moderate to severe active inflammation and/or fibrosis. However, patients with clinical evidence of cirrhosis should be treated irrespective of serum HBV DNA level.
Therapeutic goals consist of sustained remission of HBsAg from serum, accompanied by seroconversion to anti-HBs. However, this sort of outcome is rarely observed in practice, with <10% of patients attaining both endpoints.
Front-line therapy, much like HCV of times past, relies upon interferon and Viread (Tenofovir) antiviral therapy. Based on the clinical trial designs, we surmise that GS-4774 will ultimately be used in combination with Viread for 6-12 months.
According to the 2009 EASL clinical practice guidelines, the decision to treat HBV is primarily based on the combination of three criteria:
- serum HBV DNA levels,
- serum alanine aminotransferase (ALT) levels and
- histological grade and stage of the underlying liver disease.
Goals of Hepatitis B Treatment
1. Rapidly and sustainably drive viral load down to undetectable levels (antivirals such as Viread)
2. Shrink and ultimately eliminate cccDNA pools (antivirals, interferon)
3. Eliminate viral control over host immune functions (RNAi, Tarmogens, cyclophilin inhibitors)
4. Reactivate the host immune response to the viral infection (Tarmogens)




Efficacy of Available Drugs on Chronic HBV Markers

While the RNA platforms (Tekmira, Arrowhead, Alnylam) reduce expression of various transcripts, these are still unknown to stimulate a T-cell response. Further, it has been elucidated that only 1-2% of chronically HBV-infected patients annually may lose HBsAg and achieve definitive recovery; it may take decades of therapy with available treatments. Based on the fact that GS-4774’s development is being conducted by GILD, and taking into account their primary commercial strategy is the development of proprietary, best-in class regimens, we believe the market should be looking at GS-4774 in the context of how it can “boost” cure rates in combination with Viread (Tenofovir).
Following Arrowhead’s (ARWR) disappointing HBV results in October (failing to meet the consensus expectation for >1 log reduction in the HBV surface antigen) we believe expectations for >1 log reduction in HBsAg have come down, setting the stage for a favorable reception to GILD/GBIM’s data we anticipate around EASL 2015. Again, this asset is largely overlooked, thus expectations are currently nonexistent. Indeed, Alnylam’s (ALNY) management commentary confirmed earlier this year that only a >0.5 log reduction is necessary for seroconversion as long as there is a T-cell immune response in conjunction, and we reiterate that Tarmogens play a key role in “cross talk” between antigen and T-cell response, as described previously. Mechanistically, the GS-4474 Tarmogen makes more sense to be able to achieve both HBsAg reduction and stimulating a T-cell response as opposed to RNAi monotherapy. RNAi uniformly reduces the expression of the HBsAg, but is unknown to confer the T-cell immune response that may be necessary for seroconvervision; the GS-4474 Tarmogen directly interacts with HBsAg and, in theory, stimulates a T-cell response. The rationale behind the RNAi knockdown of surface antigen rests on the idea that reducing serum antigen levels will reduce the antigen barrier for an immune system that is already overwhelmed.
Lastly, it has been established that persistence of intrahepatic cccDNA at the end of treatment is predictive of off-treatment relapse, while it remains unknown how RNA drugs will perform on this metric. In our view, it is at the highest risk of viral relapse off-treatment. There also is early evidence that the predictive value of HBsAg decline may not apply equally to all HBV genotypes, a notable contrast to GS-4774 that targets homologous regions of all 3 antigens across HBV genotypes - once again Gilead seeking pan-genotypic activity.
It is conceivable that the ultimate HBV regimen will develop into a combination/sequential approach with antivirals first to reduce viral load and replication, in combination with immuno-stimulatory agents such as Tarmogens (GS-4474) that initiate a targeted T-cell response (and hopefully leading to immunity), followed by a cycles or “pulsed dosing” of antisense RNA (ISIS) or RNAi (ALNY, ARWR) targeting HBsAg to prevent or reduce the HBsAg from coming back or continuing to induce liver damage.
Indeed since the record launch and clinical success seen with Sovaldi, expectations for viral cures have risen substantially. However, despite sharing the same surname (hepatitis) and targeted organ (liver), the similarities between HBV and HCV end there. While HCV is an RNA virus, HBV is unique in its structure as a close-circular covalently linked DNA virus (cccDNA). The primary reservation we have with the RNA approach is that, in theory, it could knock-down the surface antigen but fail to ever mediate true cures, as the cccDNA could de-facto continue to reside inside the nucleus of liver cells (Tekmira may be the exception), expressing ample HBV viral RNA transcripts for the HBV core antigen (HBcAg), Hepatitis B envelope antigen (HBeAg), Hepatitis B X antigen (HBxAg), and Hepatitis B surface antigen (HBsAg) all of which contribute to the HBV life cycle. We deduce that without the ability to directly stimulate a T-cell response to clear infected cells, the long term RNA approach may not be ideal, but is likely to make the patient less infectious and less immunosuppressed. Currently, there is little evidence that this approach, as monotherapy, would deliver a true sustainable cure without a T-cell response.

Setting Expectations for GlobeImmune and GS-4774
According to the medical literature, to achieve resolution of HBV infection, the efficient recognition of the intracellular HBV antigens by the host immune cells is essential. This established doctrine drives our attention toward GBIM as Tarmogens uniquely facilitate both the “recognition” and “activation” of the host immune system toward HBV (a property that is deficient through the RNAi approach) and communicates with both CD-4+ and CD—8+ T-cells via dendritic cell activation. We think that the RNAi approach risks mistaking the correlation of clearance of HBsAg with seroconversion as causation.
According to EASL Guidelines for HBV from 2009, Chronic hepatitis B patients treated with tenofovir for up to 4 years reached undetectable HBV DNA in 96% and 99% of HBeAg-positive and HBeAg-negative cases, respectively; no viral resistance was detected for up to 4 years; HBsAg loss occurred in just 11% of cases. We believe “success” for GBIM and GS-4774 would look like one of two outcomes:
1. Reduction in time to clearance of HBsAg
2. Increase in clearance rates from 11% to 16% would represent an incremental 50% benefit above Viread’s current cure rate.
Prior to Sovaldi’s approval, real world cure rates in HCV were 40-50% with interferon and ribavirin after 48 weeks of treatment, and incrementally better with 1st and 2ndgen protease inhibitors of 60%. We resolutely remind investors that cure rates with available therapies for HBV remain between 10-15% and require chronic therapy, not acute treatment as seen with HCV. Consequently, an observable dose-response targeting HBsAg reduction would 1) validate the platform in HBV, 2) potentially serve as a backbone regimen with other drugs in development such as GS-9620 (Gilead’s TLR7 agonist), which has a mechanism of action that is very complimentary with T-cell function and theoretically with Tarmogens. Practitioners would undoubtedly view 15-20% of patients achieving HBsAG loss as success. Of equal significance would be both the time to cure and the durability of seroconversion as this would reduce damage from chronic infection (decompensated cirrhosis and liver cancer).
We also caution investors not to confuse expectations in HBV with HCV: achieving an all-oral, short duration regimen in the near future. We are in the early innings of this story, and any safe incremental improvement in time to cure or response rates above the standard of care represents a meaningful success. Lastly, there is adequate evidence in the literature supporting the mechanistic approach of GS-4774 that gives us confidence in GILD/GBIM’s approach. For example, it has been previously established that “vaccines consisting of recombinant HBsAg and anti-HBs immunoglobulins could induce HBs-specific T cells efficiently since the formation of Ag-Ab immune complexes could be easily captured and taken up by Dendritic Cells. Although HBsAg might suppress the functions of Dendritic Cells, HBsAg-pulsed Dendritic Cells might enhance an HBV-specific immune response.” Recall, Tarmogen’s first step upon entering circulating is uptake into dendritic cells through receptor-mediated endocytosis, via attachment through toll-like receptor’s (TLR’s) stimulating cytokine’s to be released. More importantly however, is that the Tarmogen carries the main HBV antigens (surface, core, and envelope antigens) that re-sensitize dendritic cells from a nascent state induced by HBV and the HBsAg.
Remember this asset is already partnered with (GILD), unquestionably the leading anti-viral innovator in the world, and we want to highlight 5 reasons for confidence in the Tarmogen platform:
- Gilead entered into their licensing deal wth GlobeImmune after and in spite of the fact that GBIM terminated an earlier HCV program, in 2010. As well, the licensing occurred after some disappointing oncology data surrounding a mutant RAS-targeted product partnered with Celgene.
- Gilead currently has more researchers working on HBV than there ever were working on HCV at the peak of development. HBV is arguably Gilead’s #1 antiviral priority now that their HCV franchise is on solid trajectory.
- Looking at the recent timelines of GILD/GBIM HBV trials with GS-4774, Gilead should have received final data collection for the primary endpoint around September from A Phase 2, Randomized, Open-Label Study to Evaluate the Safety and Efficacy of GS-4774 for the Treatment of Virally-Suppressed Subjects With Chronic Hepatitis B looking for a mean change in log10 IU/mL serum hepatitis B surface antigen (HBsAg) from Baseline to Week 24. With final study completion expected by March 2015, we expect data at EASL 2015.
- In October, GILD verified the second Phase 2 is currently recruiting, with a few notable differences in exclusion criteria from the ongoing Phase 2 trial:
- Received prolonged therapy with immunomodulators (e.g., corticosteroids) or biologics (e.g., monoclonal Ab, interferon) within 3 months of screening.
- Receipt of immunoglobulin or other blood products within 3 months prior to enrollment
- First Phase 2 in “Virally Suppressed, HBV DNA below the lower limit of quantification,” new Phase 2 “HBV DNA ≥ 2000 IU/mL.”
- In October, GILD verified the second Phase 2 is currently recruiting, with a few notable differences in exclusion criteria from the ongoing Phase 2 trial:
- Is this an ARWR Redux?
- We do not think so, as expectations are and should be entirely different based on the contrasting mechanisms of action and intended place in the treatment paradigm. Further, RNAi singularly targets the reduction of mRNA expression, and thus reduces HBsAg. While the GS-4774 Tarmogen’s role in therapy is to re-sensitize the immune system to the 3 main HBV antigens, not just the surface antigen. In the literature, it is thought that targeting both intra and extra cellular HBV antigens are critical for seroconversion.
- Primary mistake that Sell-Side analysts made was assuming early signs that an ALT flare from AR-520 was associated at lowest reduction of surface antigen, this was not in fact the case.
- Confirmed by a preclinical expert “the flare coincided with a return of HBsAg, not indicating an immune response, but inflammation due to return of the HBsAg to pretreatment levels.”
- Low safety risk: Tarmogen’s are in eleven Phase 1 and 2 clinical trials, and have been administered to more than 500 patients and healthy volunteers, including some who have received monthly dosing for over five years
GlobeImmune’s Oncology Program and Celgene
GBIM’s CELG partnership is completely under the radar: a survey of CELG sell-side research indicates that many analysts fail to even list the partnership. Also noteworthy: CELG owns almost 11% of GBIM’s total shares outstanding. Celgene, entered into a collaboration and option agreement for certain oncology product candidates in May of 2009. Under this agreement in July 2013 Celgene exercised its option for a worldwide, exclusive license to the GI-6300 program, which is a Tarmogen program targeting the brachyury protein. Brachyury plays a role in the metastatic progression of certain cancers and is believed to be fundamental in the formation of chordomas, rare bone tumors of the spine.

- GI-4000, a Phase 2 Tarmogen targeting the mutated RAS oncogene that is present in approximately 200,000 new cancer cases each year across pancreatic, non-small cell lung, colorectal, endometrial, and ovarian cancers, as well as melanoma and multiple myeloma.
- GI-6207, a Phase 2 Tarmogen targeting carcinoembryonic antigen (CEA), a protein that is over-expressed in a large number of epithelial cancers(including non-small cell lung cancer, colorectal, pancreas, breast, gastric, and medullary thyroid cancers) with an estimated prevalence of 500,000 new cancer cases in the United States each year. Critically, CEA is minimally or not expressed in healthy cells, and may be ideally suited for targeted reduction of cancer cells with minimal risk of off-target toxicity.
- GI-6301, a Tarmogen targeting cancers expressing the brachyury protein, including ~ 60% late stage lung cancer, breast, colon, bladder, kidney, ovary, uterus, and prostate cancers. Importantly, the NCI is conducting a Phase 1safety, immunology and early efficacy trial of GI-6301 monotherapy in patients with late-stage cancers known to express the brachyury protein including chordoma, a rare and difficult-to-treat bone tumor where brachyury is expressed in 100% of patients.
- Recently updated data from eleven chordoma patients demonstrated that there was one confirmed partial response by RECIST, which has continued past one year, and eight patients with stable disease at day 85 restaging (two of these patients had stable disease at study entry).
- 7/11 patients have left due to progression.
- 4/11 patients remain on study with stable disease (3/4 had stable disease at study entry). A randomized Phase 2 study in chordoma is planned to begin by year-end.
- Recently updated data from eleven chordoma patients demonstrated that there was one confirmed partial response by RECIST, which has continued past one year, and eight patients with stable disease at day 85 restaging (two of these patients had stable disease at study entry).
Capital Structure and Finances
At current prices, GlobeImmune is trading not far above its cash value, creating a favorable risk profile for investors given the cash backstop if the stock continues lower. GlobeImmune had 5.75 million shares outstanding at August 1, 7.7 million on a fully diluted basis. At $5.50, GBIM is a $42 million company.
At June 30 the company had $6.5 million in cash and netted $14.6 million from the IPO. The company will have burned in the neighborhood of $2.9 million in the third quarter, thus we estimate GlobeImmune’s cash position at September 30 at $18.2 million. This is sufficient to fund operations through 2015, according to the company, and more importantly, the upcoming HBV readout, which may be accompanied by an undisclosed milestone payment and act as an inflection point for GBIM.
Note: GBIM has 762,000 warrants outstanding at $10, and an additional 60,000 at $12.50. Full exercise would bring in an additional $8 million dollars in cash.
Additionally the company’s small float, less than 4 million at August 1, make trading in the name thin – but potentially explosive with increasing demand.
Conclusion
With the disappointment of ARWR’s data and the momentous shift in expectations in the near term feasibility of an HBV cure, we view GBIM as a rare and emphatic Buy ahead of key data, AASLD, and an earnings event, with possible news-flow from CELG in oncology by year-end.
We want to highlight that GBIM represents the antithesis of ARWR ahead of its first major data read out. In our view, sell-side research promoted aggressive expectations (yes, perpetuated by ARWR management) which was responsible for investor expectations and response to the disappointing ARWR data. Titles of sell-side research such as “On the road with big idea…we predict Street interest in Hep B just getting started” or “A Risk Worth Taking” and characterizing ARC-520 as “…RNA interference like ARC-520 could represent the HBV silver bullet “ reflect the hyped nature ahead of ARWR data.
GBIM represents the opposite scenario, with no analyst coverage at the time of this writing. After ARWR’s disappointment, the risk/reward in GBIM shares trading close to cash and low expectations of success is a clear buying opportunity on a leveraged pure-play of GILD’s HBV program. The street attributes zero value currently.
Combining fundamental analysis with technical analysis leads to our base case price target that GBIM reclaims $10 per share before HBV data is even released, using a 20% probability of success and valuing no other clinical stage assets (technically, $10 coincides with the 61.8% retracement from all-time highs). Upon a positive outcome, we see fair value at $17 per share.
However, we note that Deutsche Bank believes that in 2015 “GILD will present data for its Tarmogen & TLR-7 compounds for HBV. We expect the street to begin to assign probabilities to these revenue streams of up to 50% upon good proof of concept data.” This would imply a $36 DCF valuation. Deutsche Bank’s DCF model on GILD values HBV assets at ~$20 per share, or $30B on 100% probability of success. Assuming 1/3 to GS-4774 implies $10B and $1.3B in peak royalties.

Note that we do not model innovation or improving cure rates growing the annual treated HBV population. This growth phenomenon has very clearly been demonstrated with HCV.
To err on the side of conservatism, we model a price ~50% below interferon in HBV, at $16,000, which was determined using 1/3 modeled price of ALN-HBV asset by Leerink.

Consequently, we modestly model 20% peak market share and only 15% odds of FDA approval, despite being partnered with GILD and a Phase 2 asset, where the historical rate of success “approval” is closer to 35-45%. Given the novelty of the Tarmogen platform, we await phase 2 data in early 2015. If successful we would attribute a 50% probability of approval, raising our target price to $36 per share.
Lastly, we want to reiterate how seldom we can find a company <$100M market cap, partnered with two best of breed biotech giants, both of which continued development with GBIM after the company’s early HCV program was terminated in 2010. Recently, according to ISI Group’s March Schoenebaum, the consistent feedback is “Celgene is the best in the business for licensing deals with smaller biotech.”
References:
http://www.smw.ch/content/smw-2011-13264/
http://www.sciencedirect.com/science/article/pii/S0161589009002016
http://link.springer.com/article/10.1186/1479-5876-12-129#page-3
Disclosure: Dr. De Santis is Founder and CEO of Alpha BioPharma Advisers, a healthcare research firm ...
more


