OncoSec Medical: The Game Changer In The Biotech Industry
OncoSec is a biotechnology company that every investor should take a look at. Besides having great management with experience in the immunotherapy sector, the company may have technology that will change the way cancer patients are treated in the future. The company currently trades at $0.50 per share, and we expect it to increase in share price in 2014.
One biotech company is striving to incorporate technology that will hopefully help many patients with melanoma--skin cancer. This company is known as OncoSec Medical Incorporated (OTCQB:ONCS) , and it is developing a technology known as Immunopulse to treat these patients with metastatic--advanced--Melanoma.
It is attempting to achieve this goal by using something known as "Immunotherapy". In essence companies developing immunotherapy treatments are shying away from toxic drugs, and going more towards a toxicity free therapy. Also Immunotherapy is being developed to work in tandem with the body's own immune system. Your T-cells always protect your body, but when it comes to targeting specific cancerous cells in proliferation--cell dividing-- it has a difficult time to pinpoint what it needs to kill. This is where Immunotherapy comes in to act like a guided missile.
ImmunoPulse: A New Technology For A New Era
To attempt a go at Immunotherapy OncoSec has a technology known as Immunopulse. Immunopulse is a DNA IL-12 Vaccine antigen that is injected into patients along with another technology known as electroporation. OncoSec Medical developed their own DNA IL-12 vaccine, but the electroporation technology comes from another small-cap biotech known as Inovio Pharmaceuticals (INO). In short the CEO of OncoSec Medical Punit Dhillon used to work for Inovio. He broke away from Inovio to form OncoSec Medical. He wanted very much for his new company OncoSec Medical to have the electroporation technology. This is where OncoSec and Inovio made an agreement for OncoSec to license the electroporation technology. This license agreement was made back in March of 2011. Under the terms of the deal OncoSec had to immediately pay $250,000 dollars up front. The agreement also calls for OncoSec to pay $2.75 million dollars to Inovio by March 24, 2013. In addition to all these stipulations, OncoSec has to pay royalty fees to Inovio upon marketing any of these approved products that use the electroporation technology.
NeoPulse: An Alternative Technology For Existing Therapies
A good thing to note about the agreement is that it has a dual benefit. This is because not only did OncoSec have the opportunity to use the electroporation technology with its DNA IL-12 antigen, but that it could also use it to deliver chemotherapeutic targets as well. One such example is OncoSec's other technology known as NeoPulse. NeoPulse uses existing chemotherapeutic drugs already known to cause apoptosis--cell death--on cancerous cells. One such example is the company using Bleomycin which has already proven to cause cell death in the human body, and incorporating it with electroporation. The end result is increased cellular uptake of the Bleomycin drug component without the toxicity spilling to other cells of the body. The reasoning for the company to move on with this component is the efficacy that has already been proven in mice in pre-clinical studies. By adding electroporation with Bleomycin, scientists observed increased clinical efficacy compared to Bleomycin as a stand alone chemotherapeutic drug.
Electroporation: ImmunoPulse And NeoPulse
The technology of electroporation created by Inovio Pharmaceuticals is in itself great, because it can be adapted to many different types of vaccine technologies. By that I mean that whether it is Inovio's Syncon DNA vaccine, Immunopulse DNA IL-12 vaccine, or Bleomycin all can be used with electroporation to significantly increase uptake into the cells to increase the efficacy of the product.
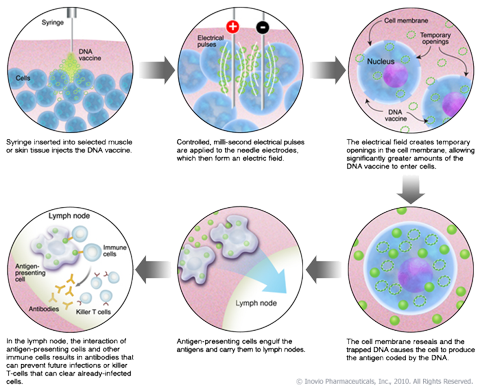
As you can see above the picture describes the steps necessary for the electroporation technology to be beneficial for the vaccine being used. For starters the needle is injected at the target site of the skin, but it is quickly followed by an electric pulse in the same area. This achieves the cells to open briefly. This pulse is the whole foundation of the technology, because the vaccine has to be allowed to get into the cells. When the vaccine enters the cell it gives it an antigen that will be used to clear the infected targeted cells. As you can see above the process involves a few steps, but the process is quickly initiated. The good part about the electroporation technology is that it has been tested for safety on many human patients. The worst to come of electroporation might be mild redness at the site of the injection. Other than that this technology is pretty safe for human use.
ImmunoPulse Phase 2 Preliminary Results
OncoSec had reported early preliminary results for phase 2 OMS-I100 back on November 2012 at the 6th World Meeting of Interdisciplinary Melanoma Skin Cancer Centres & 8th EADO Congress. These results dealt with patients with metastatic melanoma, and were pretty impressive nonetheless. The trial enrolled 25 patients with melanoma that were followed for one cycle of treatment. The results showed that 95% of the patients enrolled in the study demonstrated a response of their lesions. One key thing to note about this analysis is that it was done with 50% of the patients enrolled (13 of 25) total.
PD= Progressive Disease, SD= Stable Disease, PR = Partial Response, CR = Complete Response
| DAYS | PD | SD | PR | CR |
| 39 | 5% | 14% | 42% | 39% |
| 90 | 0% | 5% | 50% | 45% |
| 180 | 0% | 50% | 0% | 50% |
As you can see in the chart above the interim analysis shows that as the days advance the patients improve in their response to their lesions. At day 180 though the reasoning for the split between Stable Disease, and Complete Response is because there were only 2 evaluable patients at the time of testing. Overall the preliminary results showed significant of patients responding to their lesions with minimal side effects. The side effects had only made it to grade 2 -- low level-- which was related to some pain due to the electroporation portion of the treatment. The CEO Punit Dhillon had this to say in a quote
"We are encouraged to have observed at least one complete response based on the evaluable patients that have met the primary endpoint timeline, and we will continue to follow this patient and the others to assess the durability of response. These data validate the results from the Phase I study and effort we have put into this program, while providing further evidence of the potential role of ImmunoPulse as a safe and effective option for treating this aggressive disease."
In the quote above the CEO discusses the potential of this Immunotherapy known as Immunopulse to treat patients with metastatic melanoma. He states that the preliminary data seen thus far is great for patients with this severe disease. He also stated that these patients will have to followed longer to see if their responses can continue throughout the study. The company had been great in at least seeing one patient achieve a complete response, which is very encouraging for a severe disease of this form.
ImmunoPulse Phase 2 Interim Data Results
Just recently OncoSec announced Positive interim results for their phase 2 trial of OMS-I100 in patients with Metastatic Melanoma. This interim analysis concluded the final analysis of 21 evaluable patients in the study. After 180 days with treatment of OMS-I100 38.1%--8 of 21 patients--achieved an objective overall response. An objective overall response indicates that these patients responded to the treatment with either a complete response or a partial response. In conclusion 6 patients--28.6%--achieved a partial response to this treatment, and 2 patients--9.5% achieved a complete response to the treatment. Investors should note the 2 patients achieved a complete response as this indicates complete regression of the tumor. The way these results was measured was by using something known as the RECIST criteria. That means that objective overall responses of the patients to the treatment were measured by the ability for the tumors to shrink by an amount of equal to or greater than 30%. These results were presented by the lead investigator of the study Adil, Daud M.D. at the Advances in Cancer Immunotherapy meeting at the University of California San Francisco. The chief medical officer of OncoSec, Robert H. Pierce had this to say in a quote:
"Systemic response is significant for two main reasons. First, it suggests that unlike most locally administered melanoma treatments, ImmunoPulse may induce antitumor response throughout the entire body, which would have clear benefits in the treatment of metastatic disease. Secondly, the favorable safety profile of ImmunoPulse indicates its potential to deliver systemic benefit, without the toxicities associated with many other systemic treatments. We are therefore highly encouraged by this finding, combined with the safety and primary efficacy data, and look forward to continuing our investigation of OMS-I100 in the treatment of metastatic melanoma."
As we can see from the quote above this clinical trial was key to the success of the OMS-I100 vaccine. This vaccine was able to produce enough systemic efficacy without causing system toxicity like all other current treatments for Metastatic Melanoma. Also the proof of the efficacy was for the vaccine to be able to produce systemic exposure to the entire body. This systemic exposure thus proves a far better treatment for Metastatic--advanced--cancers in the human body. This is because Metastatic diseases progress throughout more parts of the body besides one intended target. The ability for this vaccine to not only be targeted against the tumor of choice, but to be able to eliminate tumor cells in other parts of the body is remarkable.
Additional Clinical Trials
Besides OncoSec having its lead vaccine in production for Metastatic Melanoma, the company is working on two other clinical areas.
- OMS-I110 (Merkel Cell Carcinoma) - A rare and deadly disease with a mortality rate of 40%. Key factor is that 80% of patients that obtain this disease get it through a virus infection. A current phase 2 trial is currently running in this open-label, multi-center trial that will enroll up to 15 patients total
- OMS-1120 (T-cell Lymphoma)- is a disease characterized by a rare form of non-Hodgkin's lymphoma that affects the patients T-cells. This infection of the T-cells results in a weakening of the immune system causing a host of other problems. This phase 2 trial is open-label as well, but is only recruiting 27 patients in one center in the United Sates
Management
There are a couple of good management members for Oncosec, but I will discuss one first in particular who has been able to take Inovio's electroporation device, and apply it to DNA vaccines with much success. This man is known as Punit Dhillon the CEO of Oncosec Medical. As mentioned above he was able to license the technology from Inovio known as electroporation, and incorporate their own DNA IL-12 vaccine to it. Much of today's success for OncoSec is the ability of the CEO to combine the two technologies together. Punit Dhillion used to be Vice president of Operations and Finance at Inovio Pharmaceuticals, before breaking off and forming Oncosec Medical.
Another member of management that will add success for the company is the Chief Medical officer known as Dr. Robert H. Pierce. Dr. Robert has extensive clinical experience in immunotherapy companies thus the interest in joining OncoSec. He used to be an executive director/member of the global Anti-PD1 Development Team. PD1 is a current immunotherapy being developed at other big pharmaceutical companies currently such as Merck (MRK) and Bristol-Myers Squibb(BMY). Merck was able to show some amazing results in their drug known as lambrolizumab--MK3475--which was showing better efficacy results than Bristol-Myer's drug nivolumab. Also showing some amazing results in the area of PD1 drugs for cancer immunotherapy is Roche(OTCQX:RHHBY). With the backing of this Chief Medical Officer with extensive experience in the immunotherapy area at Merck, we believe OncoSec management is the one to place the company at the top with the big pharmaceutical companies in the future.
Financials
According to the 10-Q SEC filing OncoSec has cash of $15.2 million dollars. This amount of cash on hand is expected to give the company room to run for at least 12 months in which time it will have to find other financing options way before that period. Such financing options would include the ability for the company to raise more cash through a share offering, or some form of debt. Ideally though as we have shown thus far OncoSec's Melanoma results have been outstanding, so it is possible for the company to form a partnership with a big pharmaceutical company for ImmunoPulse. Such a deal would alleviate the declining cash position, and allow the company to continue to run all of its current clinical trials. Although in the event the company has a hard time finding new forms of financing it may have to scale back its additional clinical trials in other areas. Such scaling back of clinical trials to go would be the Merkel Cell Carcinoma, and T-cell Lymphoma trials.
As mentioned above OncoSec came to an agreement to license certain electroporation technology from Inovio Pharmaceuticals. In doing so OncoSec still owes at least $1,000,000 dollars in its obligation to pay back Inovio by December 31st. The company will have to dilute to keep the company operating way before the 12 month period to make sure that its operations remain intact. Such an effort to obtain financing was back on September 18th of 2013 when the company sold 47 million shares of its common stock, and the purchase of warrants of 23 million shares. With this offering OncoSec was able to establish $11.1 million dollars to use to fund its clinical trials. Investors will definitely feel some short term effects from more dilution coming up, but considering how the clinical trials have been coming the long term outlook seems refreshing.
Risks
There are some risks that are associated with investing in a small-cap biotechnology stock:
- Early efficacy has been seen in a small subset group of patients, but may not translate into bigger pools of patients
- Efficacy seen thus far has been substantial for phase 1 and phase 2 but may not meet its primary endpoint in the phase 3 clinical trial
- Whole platform is dependent on DNA vaccines, and electroporation so a big failure in one trial would mean a bleak future on the other clinical trials
- Financing may be difficult to come by thus the company will have to cut some clinical trials to meet its current obligations
- OTC stocks carry more risks, and a future reverse split may be necessary to uplist to a bigger exchange in the future reducing investor's shares
- There is no guarantee that future catalyst results will create big surges on the share price
- There is no guarantee that the company will be able to form a partnership with another big pharmaceutical company to run the clinical trials
- Even upon approval there may be a lot of competitors on the market with other types of immunotherapeutic products, which would mean substantial competition going forward
Conclusion
OncoSec has figured out a more safer and effective way to treat patients with DNA vaccines--licensed electroporation. If the platform proves to be successful in a final clinical outcome in phase 3 then this small cap biotechnology company will for sure be a game changer in the biotech industry. Such positive results for Melanoma patients would mean safer forms of treatment, and substantial rewards for investors. We think with further clinical evidence in 2014 we should see OncoSec's share price rise in 2014.
I am Long ONCS, no position in other stocks mentioned