MS An Interesting Upshot Opportunity For RedHill's RHB-104
TM editors' note: This article discusses a penny stock and/or microcap. Such stocks are easily manipulated; do your own careful due diligence.
On December 12, 2016, RedHill Biopharma Ltd. (Nasdaq: RDHL) reported final results from the recently completed Phase 2a clinical study examining RHB-104 as an add-on therapy to interferon beta-1a for the treatment of relapsing-remitting multiple sclerosis (rrMS). This is the second indication for one of RedHill's most important clinical-stage candidates. RHB-104 is currently in a Phase 3 clinical trial for the treatment of Crohn's disease. Below is a quick review of RHB-104 and some thoughts on the Phase 2a data in rrMS.
Quick Review of RHB-104
RHB-104 is a proprietary and potentially groundbreaking oral antibiotic combination therapy with potent intracellular, anti-mycobacterial, and anti-inflammatory properties. Development programs with RHB-104 include the Phase 3 MAP U.S. study in Crohn's disease (CD) and the recently completed Phase 2a exploratory study in rrMS. The development of RHB-104 is based on increasing evidence supporting the hypothesis that inflammatory / autoimmune diseases like CD and MS are (in part) caused by Mycobacterium avium paratuberculosis (MAP) infection in susceptible patients.
For a detailed look at the science behind RHB-104, see my article from July 2016:
→ RedHill's Phase 3 Crohn's Drug Is A Potential Game-Changer
RedHill is currently enrolling patients in a Phase 3 clinical study called MAP U.S. at up to 150 clinical sites in the U.S, Canada, Europe, Israel, Australia and New Zealand. Target enrollment for the study is 410 patients. The company announced an expansion of this program in October 2016 and just announced this morning that the first DSMB review has been cleared. This is the first Phase 3 study conducted by the company with RHB-104.
Encouraging Phase 2a MS Data
The Phase 2a study with RHB-104 in rrMS, dubbed CEASE-MS, was a single-arm, open-label study designed with a series of exploratory endpoints to evaluate the safety and potential efficacy of fixed oral dose RHB-104 as add-on therapy to interferon beta-1a for 24 weeks. The CEASE-MS Principal Investigator was Dr. Radi Shahien, MD, of Ziv Medical Center in Safed, Israel. The trial took place at two sites in Israel and enrolled a total of 18 subjects. Subjects were followed for a total of 48 weeks, the second 24 weeks taking only interferon beta-1a.
The primary endpoint was the change in number of combined unique active (CUA) lesions during the 24-week RHB-104 treatment phase. Secondary endpoints included the change in number of CUA lesions during the post-treatment 24 to 48 week time period, number of relapses, analysis via the expanded disability status scale (EDSS), MAP infection status, and safety. Top-line results were released in late March 2016.
Final results released earlier this week were consistent with the previously announced top-line data, suggesting meaningful positive safety and clinical signals upon 24 weeks of treatment with RHB-104. In short, the marked improvement over historical and baseline disease levels seen after only 24 weeks of RHB-104 seemed to be maintained after discontinuing the drug, suggesting that RHB-104 had a disease modifying effect. This would be expected if MAP infection does play an underlying role in MS pathophysiology. Further analysis of MAP infection status remains ongoing; however, a high correlation between MAP eradication and disease stabilization bodes incredibly well for RedHill's RHB-104 hypothesis.
A total of 18 patients were enrolled in CEASE-MS; however, one patient violated protocol by taking outside medication during the dose-escalation portion of the trial, so only 17 patients were included in the analysis modified intent-to-treat (mITT) analysis. All 17 patients had been treated with interferon beta-1a for an average of approximately five years prior to enrollment in the study, experienced at least one MS relapse within 12 months prior to enrollment or two MS relapses within 24 months prior to enrollment, and had an EDSS score of 6.0 or less at screening (mean of 3.06). The per-protocol (PP) analysis included ten patients, all of whom completed both the dose escalation and 48-week study period without any major protocol deviations.
- The Results -
- The annualized relapse rate (ARR) at 24 weeks was 0.29 in the mITT population and 0.0 in the PP population. Notably, the ARR remained 0.0 in the PP population throughout the 24-week post-treatment period as well. The 0.29 ARR number in the mITT group compares favorably with previously reported pivotal studies of interferon beta-1a therapies Avonex® (0.67)(1) and Rebif® (0.87-0.91)(2). The data also compares well to other first-line therapies, as referenced in Nature, 2011 (3).
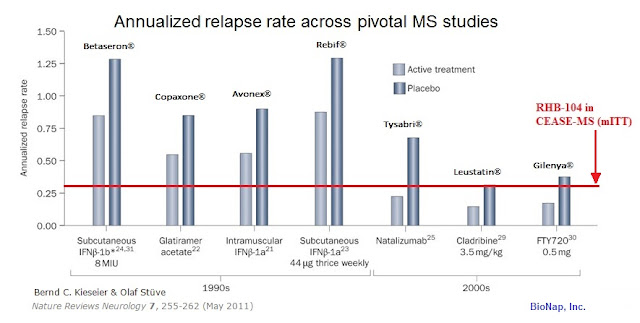
- Importantly, 100% of the PP patient population was relapse-free through the entire 48-week study. For the mITT population, 88% was release-free during the first 24-week treatment period and 93% was relapse-free during the 24-week post-treatment period. These data compare favorably with previously reported pivotal data on the use of Rebif® (75%) in comparison with Avonex® (63%) as standalone first line therapies (4). The 93% number is also superior to roughly 80% that can be expected on Tysabri monotherapy (5). Additionally, the company reported no patients in the CEASE-MS study relapsed after week 8 of treatment.
- RedHill reported the Expanded Disability Status Scale (EDSS) scores, a standard measure of MS disability, showed signs of disease stabilization. Deterioration during the period off RHB-104 was associated with a change in ambulation in one patient. Importantly, no increase in total EDSS scores was observed in any of the patients while on treatment and no patients experienced a decrease in total EDSS scores once off treatment.
- Burden of disease was defined as the total volume of all T2 lesions. A T2 lesion in this context is defined as a new or enlarging lesion or a lesion reappearing at a site of previous lesion resolution. Data published in The Lancet in 1998 notes that T2 lesion load is expected to increase by approximately 11% per year in rrMS patients if left untreated (6). Although not powered for efficacy and conducted over a short period of time in a small number of patients, the CEASE-MS study results indicate a reduction in T2 lesion volume at 24 and 48 weeks of treatment with RHB-104 as compared to baseline, suggesting reduction in MS disease burden and comparing favorably with previously reported Avonex (7) and Rebif (8) data.
- The primary endpoint of the study was the number of combined unique active lesions (CUAs) in the total population. Among all patients followed for 48 weeks, only five active T1 post-gadolinium lesions were noted. CUAs in the study were almost entirely driven by changes in T2 lesions, and changes in total CUAs at weeks 24 and 48 were not clinically significant.
- Finally, RHB-104 was found to be safe and well tolerated during the 24-week dosing period, with no drug-related serious adverse events (SAE) or other clinically relevant or unexpected adverse events noted. This is important and bodes well for the ongoing Phase 3 trial with RHB-104 in CD noted above.
- Analysis of MAP infection rates is still ongoing.
Next Steps With RHB-104
MAP, an obligate pathogenic bacterium that undoubtedly plays a role in gastrointestinal inflammatory conditions, such as Crohn's, may also be involved in MS disease pathophysiology. If RHB-104 worked to eliminate an active MAP infection in these rrMS patients, which we will know more about in the coming months, then RHB-104 may have a disease-modifying effect for patients with MS. This would present the potential to incorporate RHB-104 into the standard-of-care for patients with rrMS and confirmed MAP infection. RedHill is working on a companion diagnostic to detect MAP infection in identify patients that might benefit from RHB-104 treatment.
With positive interim Phase 3 data in CD and demonstrated proof-of-concept in MS, I believe RedHill will have a very good chance at signing a lucrative development and commercialization agreement for RHB-104. Management's goal right now is to generate as much data as possible with RHB-104 (note: management may also initiate proof-of-concept work with the drug in rheumatoid arthritis). The more data that RedHill can generate around RHB-104, then potentially the more partners management can bring to the table for talks around a deal.
Conclusion
In the meantime, the company continues to be well-funded, having exited the third quarter 2016 with $40.5 million in cash in the bank. RedHill has numerous catalyst on the horizon that should make the next six to nine months a good time to be a shareholder, including the potential closing a product acquisition or in-licensing in the next few weeks. The anticipation is that this transaction will be for a commercial asset that will potentially generate revenues and catapult RedHill into a fully integrated U.S. specialty pharmaceutical. In the meantime, my sum-of-parts valuation pegs the value of the company at $500 million, an increase of roughly 250% from today.
Disclosure: Please see additional important information about BioNap, Inc. in our Disclaimer.
We hold no ...
more


