Johnson & Johnson Under Pressure As FDA Calls For Pause On COVID Vaccine
Shares of Johnson & Johnson (JNJ) are under pressure on Tuesday after the Food and Drug Administration and the Centers for Disease Control and Prevention recommended a pause in the use of the company's COVID-19 vaccine following blood clotting cases. On the flip side, competitor Moderna's (MRNA) shares are on the rise, with the company saying that the totality of the available safety data for its COVID vaccine "does not suggest an association with cerebral venus sinus thrombosis or thrombotic events".
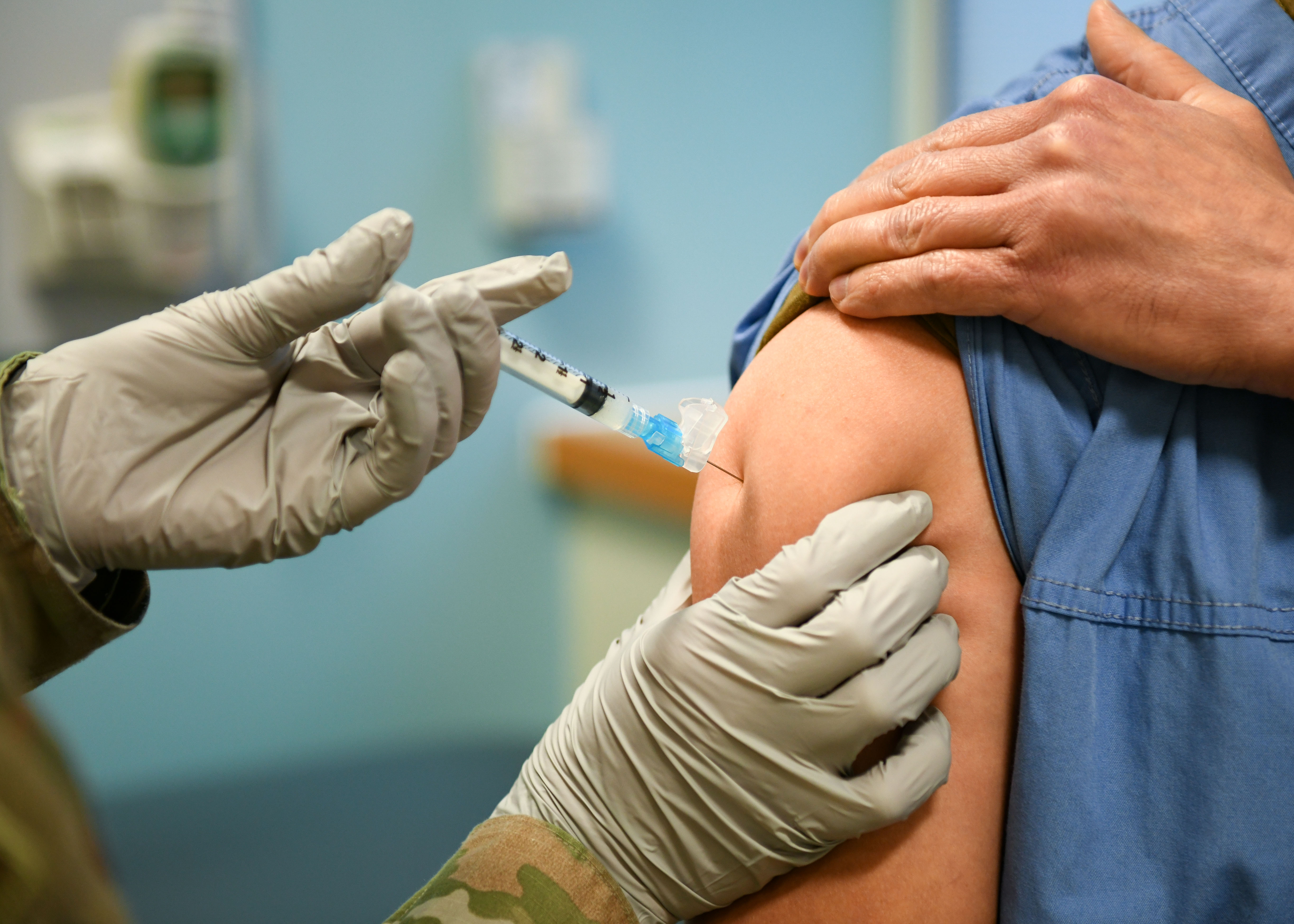
PAUSE ON J&J COVID VACCINE: In a joint statement, CDC and FDA said that "As of April 12, more than 6.8 million doses of the Johnson & Johnson vaccine have been administered in the U.S. CDC and FDA are reviewing data involving six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving the J&J vaccine. In these cases, a type of blood clot called cerebral venous sinus thrombosis was seen in combination with low levels of blood platelets. All six cases occurred among women between the ages of 18 and 48, and symptoms occurred 6 to 13 days after vaccination. Treatment of this specific type of blood clot is different from the treatment that might typically be administered. Usually, an anticoagulant drug called heparin is used to treat blood clots. In this setting, administration of heparin may be dangerous, and alternative treatments need to be given. CDC will convene a meeting of the Advisory Committee on Immunization Practices on Wednesday to further review these cases and assess their potential significance. FDA will review that analysis as it also investigates these cases. Until that process is complete, we are recommending a pause in the use of this vaccine out of an abundance of caution. This is important, in part, to ensure that the health care provider community is aware of the potential for these adverse events and can plan for proper recognition and management due to the unique treatment required with this type of blood clot."
Meanwhile, Johnson & Johnson confirmed that it is "aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. […] We have been working closely with medical experts and health authorities, and we strongly support the open communication of this information to healthcare professionals and the public." The company is also "proactively" delaying rollout of its vaccine in Europe.
New York will replace Johnson & Johnson's COVID-19 vaccines with the Pfizer (PFE) vaccine for appointments scheduled for Tuesday, Orion Rummler of Axios reported, citing New York's health commissioner Howard Zucker.
'INCREMENTAL NEGATIVE': Commenting on the news, JPMorgan analyst Chris Schott said he views the recommended pause of Johnson & Johnson's COVID-19 vaccine based on six reported cases of severe blood clots as an "incremental negative" for the company. At the same time, the adverse event rate appears extremely infrequent and potentially manageable, the analyst contended. Schott also sees Tuesday's update as an "incremental positive" for Pfizer's BNT162 vaccine. More broadly, the pause will have a limited earnings impact on J&J given non-for-profit pricing during the pandemic, he added.
MRNA VACCINES: Meanwhile, Morgan Stanley analyst Matthew Harrison told investors that he sees continued news around potential safety issues with viral vector vaccines improving the outlook for mRNA vaccine makers for 2022 given that marginal demand could shift toward mRNA vaccines from Moderna and Pfizer. He does not expect the overall U.S. vaccination effort or timeline to be materially impacted, even if the pause in use of the J&J vaccine is significant.
Following J&J news, Piper Sandler analyst Edward Tenthoff reiterated an Overweight rating on Moderna with a $208 price target on the shares. The analyst believes Moderna's two-shot mRNA-1273 vaccine deliveries will continue to grow to up to 1B doses this year and 1.4B doses in 2022. Tenthoff anticipates updates including potentially TeenCOVE data and more details around the booster and variant strategies at Moderna's virtual vaccine day on Wednesday. Overall, he believes Moderna is "effectively transitioning into a profitable, global commercial company."
WHAT'S NOTABLE: In a statement on CVST or Thrombotic events, Moderna said, "A comprehensive assessment of the totality of the available safety data for mRNA-1273 after over 64.5 million doses administered globally does not suggest an association with cerebral venous sinus thrombosis or thrombotic events."
PRICE ACTION: In morning trading, shares of Johnson & Johnson have dropped almost 3% to $157.25, while Moderna's stock has gained over 8% to $150.68.
Disclaimer: TheFly's news is intended for informational purposes only and does not claim to be actionable for investment decisions. Read more at more



