CytoDyn's Update Provides A Clear Path Towards Approval With Up-Listing Potential Still In The Cards
Investors were poised to hit the sell button on CytoDyn Inc. (OTCMKTS: CYDY) after their investor conference call Wednesday. It played right into the hands of the shorts and gave them one more chance to sow confusion before an impending run-up happens on good data as severe to critical read-outs are disclosed. This latest conference call pretty much cemented a potential approval in Severe to Critical COVID-19 within a month. What disappointed momentum traders was the FDA’s guidance to hold off on the CD-10 Mild to Moderate COVID-19 Emergency Use Authorization (EUA) before having a look at the interim readout on the Severe to Critical patients in the CD-12 trial. Recently, biotech investors in CYDY seem to have forgotten that clinical trials normally take years. During the call, the CEO of Cytodyn, Dr. Pourhassan tried to interject a dose of realism that the first patient was dosed on March 31 which was only 6 months ago, but assured investors there should be an approval by the end of the year.
“We have a powerful drug called leronlimab. We believe it will change the history of treatment. . .We are going to be getting approval.No matter what. That's what we believe. Because we have the data.” - CEO Nader Pourhassan
Panel of Doctors and Researchers Stake Reputations - The Drug Works
The conference call started off with doctors giving a testimony about their experience with patients in the COVID-19 clinical trials. The doctors gave powerful case studies that highlighted a drug that, in their minds, worked really well.
Dr. Harish Seethamraju
- Submitted manuscript for the first 11 eIND’s
- Lead Author in Manuscript for CD-10 almost complete
He talked about the selection of his patients and how they were not eligible for any trials. His hospital had many options but these patients were in such bad shape that “we thought were not going to be around in a day or two.”After administering leronlimab they turned around immediately and continued to recover. The things that surprised him was that patients recovered so quickly that they were able to take them off life support. He talked about end-organ recovery. He didn't think they would recover to the extent that they wouldn’t need life support anymore. He saw patients come off the ventilator and dialysis.
“Stopping inflammation and promoting immunity to clear up the virus and Institute mechanisms that preserve end organs like without pulmonary fibrosis and or renal failure. These were my observations and I was very eager to try in phase 2 and phase 3.”
Dr. Otto Yang - Dr who oversaw the treatment of Samantha Mottet - the woman who on TV claimed leronlimab saved her life.
- Submitted for publication (30 patients eIND)
Dr. Nicholas Agresti - Internal medicine, Gastroenterology, Liver transplant doctor
- Manuscript being reviewed for publication(4 patients with remarkable recoveries)
The discussion revolved around 4 patients that were in a critical condition and all on mechanical ventilators and ended up having remarkable recoveries after being given leronlimab. Half of his patients (2) were on hemodialysis. Most of his patients saw clinical improvement within 24 hours. His team thought that these patients had zero chance of survival. He said, “these people were expected to pass on.”They had received multiple treatments before trying leronlimab. In terms of cytokines he observed a decrease in IL-6 and CRP that was correlated to the dosing of leronlimab. Of the 2 patients on hemodialysis one was off by the time she was discharged and the other was on it intermittently until he recovered.
“Our patients were critically ill. These patients were even before covid19 would be patient you would likely contact the family members and ask them to come in and pay their respects because that patient would pass on. Until we have clinical trial results from phase 3 we are cautiously optimistic. Given the fact that all 4 patients recovered and received multiple other treatments and did not improve until receiving leronlimab gives us promise.”
Dr. Recknor’s comments were primarily related to the CD-10 trial. When he recruited patients it was actually a very diverse group so he learned a lot from it. Half the group ended up being mild. Enrollment didn’t exclude people who were sick a long time. Other studies frowned upon that and were looking to recruit people that had symptoms less than 10 days. He indicated that some patients in the study might have had COVID-19 four weeks before they were enrolled. He also noticed groups of patients that would just not get better due to adverse events. Dr. Recknor is coming onto the leronlimab’s team and provided huge contributions in terms of getting people enrolled in Cytodyn’s trials.
“What I was extremely impressed by is that Leronlimab seems to work extremely well beyond the 10 day time period. Some of the patients might have had COVID diagnosis 4 weeks before enrollment in the study yet still included per the protocol.”
FDA and MHRA Emergency Use Authorization
The most germane news on the call was that both the FDA and the MHRA wanted to see interim data from the Severe to Critical (CD-12) patients before ruling on the Mild to Moderate. There was a clear sense that the door was still very open on the Mild to Moderate approval in the UK and closed with respect to the USA FDA. The MHRA echoed the FDA’s sentiment that Mild to Moderate patients are not really in danger and that a look forward to the severe to critical patients that would likely chart the pathway to approval.
The MHRA however, took the discussions to a higher level than the FDA. They showed interest in the data from the 60 eIND’s. By complying with their request, early access seems like it's on the table. Early access would result in higher usage in the United Kingdom allowing the regulators to monitor results in country and get a better comfort level with using the drug. The company submitted its paperwork and is trying to get funding for the trial and patient recruiting assistance directly from the government. This would be part of the package called an Early Access to Medicines Scheme, or EAMS, which would be sponsored by the U.K.’s Department of Health and Social Care. Patients who are hospitalized with severe disease will be eligible to receive the drug. The arrangement is similar to the EUA that the U.S. Food and Drug Administration granted earlier this month and a similar one in Japan.
(Click on image to enlarge)
Mild to Moderate Trial Update Twisted by Shorts
In the moderate trial, almost half the control and half the leronlimab arm were composed of asymptomatic patients. According to protocol those with a symptom score over 4 showed clinical improvement. The breakdown can be seen in the chart above. The company crunched the numbers with statisticians and figured that they could do a moderate trial with 100 patients and get the statistical relevance they need for approval. The company seems ready to finalize their phase 3 and get started, but there is a chance they might be able to get a label extension.
After a statement from the CEO Nader Pourhassan updating the status of an EUA for Mild to Moderate, the chat boards lit up in controversy. Here is the excerpt that started it all.
“Let’s talk about the FDA and CD-12. We just said they wanted to see those results. We did not submit a formal letter to the FDA saying we want to get Emergency Use Authorization. We asked them for their opinion and they were not positive about it. Their reasoning made a lot of sense to us.” - CEO Nader Pourhassan
Many in social media pointed to the press release on August 17th that stated they filed for an Emergency Use Authorization. Adam Feuerstein was first to cry foul and tweet that they filed a “formal EUA request for leronlimab to treat COVID-19 patients” weeks ago.
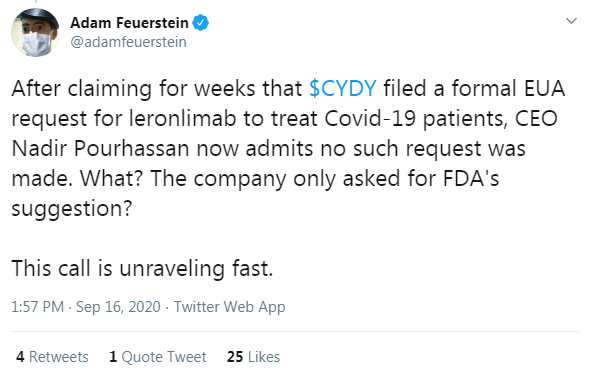
The facts show otherwise.CytoDyn never said they filed a “formal” request. There is no mention of a filing anywhere. Maybe they sent a formal request to Mexico, the UK, and the European Union. Perhaps this is why it was separated out on the press release the way it was. The company did say they were going to talk to the FDA about it and they must have otherwise how would they have come to the conclusion that filing might not be a good idea.
The company never characterized their requests as formal or informal; these are just semantics. What the public does know is that something was sent to the FDA, probably a letter, because they eventually responded and appear to be locked into some negotiations regarding a follow on phase 3 moderate trial. The public also knows that the FDA took about 3 weeks digesting the data before they responded. Looks like Adam Feuerstein needs to brush up on his reading skills.
The FDA’s rationale was that the Mild to Moderate was not their sweet spot on the treatment curve. The optimal patient population to benefit from leronlimab is the severe to critical patients.Dr. Kush Doty also made a point of this in the call that not only the FDA but also the MHRA needed more data before they made a decision. He astutely pointed out that no one in the world has an EUA or equivalent in the Mild to Moderate indication. It should be clear to investors that the Mild to Moderate EUA was a Hail Mary pass. Investors who banked on it as a given likely precipitated the sell-off because they don’t understand the role it played in the negotiations with regulators.
The FDA postulated that if the data was good in severe to critical patients, then label expansion could happen. To that end, the company has been working on a phase 3 trial design with the FDA using moderate patients that have a clinical symptom score over 4. They are looking to do a 100 patient study in Moderate COVID-19 patients and approval looks likely since they got clinical improvement of 90% on leronlimab versus 71% in placebo. The question is when will CYDY pull the trigger and announce the Phase 3 Moderate study. If they were going to accept the same protocol they could start immediately, but perhaps they are negotiating to get NEWS2 as the primary endpoint to eliminate any risk of failure. Big pharma uses these tactics, why shouldn’t CYDY stack the decks in their favor as well?
Severe to Critical Trial
Pourhassan seemed to indicate if they crush their primary endpoint the FDA is willing to grant an EUA but if they don’t they are simply going to have to finish the trial. The interim read out is for 195 patients and September 23rd represents 28 days after the last patient was enrolled. The study could be unblinded on the 24th of September. Enrollment stands at 210, but the addition of the U.K. into the mix will help dramatically. During the call Dr. Kush Doty the head of Ameraxand CytoDyn’s CRO said “site selection has started in the UK and we expect to start enrollment very soon.”Another driver of enrollment in the CD-12 trial was that eIND’s were no longer allowed by the FDA.
The trial seems to be progressing, but the company indicated that most of its energy was focused on the interim readout which could be unblinded in less than a week. It appears that many regulatory agencies, investors, and even big pharma are waiting to see the readout.
“They are talking to us for phase 3 moderate and they want to see the CD-12 results. They won't hold back on the CD-12 if we hit the ball of the park - we don't know if we are going to have to finish the trial.” - CEO Nader Pourhassan
Management and the scientists clearly believe that the CD-12 trial is going to smash its primary endpoint which happens to be 28-day all-cause mortality. The CEO makes the point that if they smash it there is a good chance they will ask for an EUA. If they are just okay results then they just have to finish enrollment so that they can hit their endpoint and ask for approval.
Blood Brain Barrier
Science is not something that people seem to get excited about but the Dr. Kelly came out with some astonishing news regarding the blood-brain barrier (BBB) and leronlimab.
“The blood brain barrier can be compromised especially with neuroinflammation. In addition, literature has shown that rantes (CCL5) can disrupt the blood brain barrier. We have just learned that through a recent study in Macaques and HIV, that leronlimab does in fact cross the blood brain barrier and has been identified in brain tissue. Even more compelling about the story is that we were seeing essentially 100% receptor occupancy by leronlimab peripherally and 70-75% receptor occupancy in the brain with leronlimab 700mg dose administered once weekly. This allows us to have further confidence that leronlimab will have the opportunity to be successful in various central nervous system pathologies including HIV associated neurocognitive disorder, MS, CNS tumors, Alzheimers, and the recovery for strokes and traumatic brain injury.”
The conference call delivered a lot of information but it might take time to process the fact that a drug that clearly has an effect in cognition as anecdotal as it may be at this point was able to make it through the blood-brain barrier. The implication of this finding is far reaching because this is equivalent to the basket trial for cancer. A number of central nervous system diseases and traumas could be potentially treated with leronlimab.
Dr. Pourhassan shared an anecdotal story about his mother-in-law who has been on leronlimab for her metastatic breast cancer since November 2019. Her disease is stable, especially the tumor metastasis in the brain. She recently suffered a stroke and her whole side was paralyzed and based on her underlying cancer the doctors told Dr. Pourhassan that she would never walk again. The next day she walked and he videotaped it. There are some journal articles that explain what appears to be a miracle. During a stroke CCR5 levels in the brain skyrocket and remain elevated for weeks. It hampers recovery so blocking it could have a very therapeutic approach. So in Dr. Pourhassan’s story theory meshed up with reality. Had his mother-in-law not been on leronlimab and blocked CCR5 upregulation in the brain she might have not recovered at all. Could this be the new PREP for stroke?
Long Hauler Study
Dr. Recknor gave the synopsis and is also the person leading the long hauler study. He observed CD-10 patients first hand in his clinic and noticed that some had a cognitive fog that resulted in them bumping into things and had extreme insomnia as well as a host of other neurological issues. There was a record of this behavior in some cases weeks before being admitted. The patients on leronlimab saw cognitive improvement. Here is an excerpt of Dr. Rectnor’s presentation.
“Patients that entered CD-10 had symptoms for weeks. I guess you could say they were kind of going into that long hauler phase but in these patients we were seeing marked changes cognitively. Fatigue resolved.I think that's a game-changer and that opens up the door to multiple neurocognitive issues.” - Dr. Chris Recknor
No drugs have been proposed in this indication, and there is very strong anecdotal evidence in the CD-10 trial of cognitive changes from the use of leronlimab. It’s widely expected that the company will get the green light from the FDA to proceed in this indication. If the FDA labels this as an unmet medical need that will lower the hurdle with respect to the amount of time needed for drug approval. Trial initiation could be a significant catalyst and it represents yet another indication in COVID-19.
NASH Clinical Trial
The NASH trial seems to be firmly underway with specific milestones established. Most of the dosing sites have been selected and are awaiting test kits due to arrive in mid-October. During the call, Dr. Kelly reiterated the size and scope of the NASH market.
“So we plan on having the first patient injected around mid to late November. We will be announcing our first patient injection to the public. But I do want everyone to understand that we believe the market opportunity for NASH for Leronlimab is tremendous. It is anticipated to be a $20B industry by 2025. ”
The NASH indication is an unmet medical need and has blockbuster potential because it is the most prevalent disease in America and has reached epidemic levels. The following other NASH companies like Madrigal (MDGL), Galectin Therapeutics (GALT), and Intercept Pharmaceuticals (ICPT) are among the seasoned names of NASH pure plays. All of their studies are of long duration, but CYDY has a chance to change the paradigm with a short and effective proof of concept trial.
Nasdaq Uplisting
Nasdaq uplisting is likely to be the next catalyst. The CEO said, “We are doing everything possible.” The company indicated that they made two voluminous submissions to Nasdaq and expected their response “sometime next week.”They were prepared to meet Nasdaq’s $4 million minimum equity threshold and have engaged a major investment bank on standby to assist. The CFO mentioned that Nasdaq was also looking for a “seasoning of the price at or above $4.00.” They have made their investment preference known to Nasdaq that they prefer to raise money later at higher valuations. The going concern issue appears to have been handled via direct correspondence between Nasdaq and the auditor. The CFO ended by saying the company is hopeful and that they will be responsive to any additional requests from the exchange.
Many investors immediately assumed by next Friday they would get feedback and the uplisting might go another round, but this conference call was set last week and the structure of the call seemed to lend itself to talking about a Nasdaq uplisting announcement that was delayed. This is complete speculation but the last announced shareholder update had news released right at the close. This time no news came and the closest catalyst is the Nasdaq uplisting. Then there was the seasoning comment about the stock staying at a specific level. It just looks like news got delayed a week later and the company was simply overzealous in expecting to provide an update during this call. If this happens, then the Nasdaq's response would potentially be early next week with a Wednesday or Thursday announcement. Many investors selling might miss out on an uplisting.
The IMMU comparison
Critics have pointed to recent $21 billion Gilead Sciences (GILD) deal with Immunomnedics (IMMU) as a sign of the company’s failure to find big pharma partners willing to invest in the technology. Pourhassan indicated that managing different indications that would have different price points like HIV and cancer makes discussion with drug partners much more complicated.
Dr. Kelly pointed out that the deal is actually a net positive for CytoDyn shareholders because he believes that immunotherapy is the future of cancer treatment and that leronlimab is on that cutting edge. He pointed out that this was the first drug approval Immunomedics has seen in 37 years and that in 2016 they were almost purchased for $2 billion but somehow managed a $21 billion purchase price 4 years later in spite of a Complete Response Letter and questions regarding their data integrity. Immunomedics drugs are essentially chemotherapy with many side effects like neutropenia (64%), nausea (69%), vomiting (49%), and diarrhea (63%).
“If we show efficacy, which product would you choose? A once a week subcutaneous injection or an IV infusion; a product that doesn’t have any strong safety signals in over 1000 patients or a product with known side effects. Cancer treatment is toxic and people are looking for medications without drug interactions, limited side effects, and ease of administration.” - Chairman Dr. Scott Kelly
It’s pretty obvious to the street that GILD overpaid for IMMU. With a total available market of only 7,500 3rd Line Triple-Negative Breast Cancer (TNBC) patients it represents only $75 million a year assuming a $100K treatment cost. Doing some simple math this represents a 280-year payback. Gilead’s CEO said it's a bit of a product in a pipeline. So clearly GILD sees platform potential and feels it could ramp up more indications. Kelly clearly wanted to make the case that immunology is the future, not toxic chemotherapy as GILD envisions, so if you had two identical products as you have in this case which would you choose? Should CYDY get approval in the same indication, Dr. Kelly’s observation reinforces just how valuable leronlimab is.
Investment Summary
Investors need to get real with their expectations. An Emergency Use Approval was not by any stretch of the imagination baked into the stock price. The value of an EUA approval was set when Gilead went up $10 billion in market capitalization on news that they might have a therapeutic that works. That represents approximately $20/share in CytoDyn capitalization for just and EUA approval. Assuming a $4 price and that investors were giving no value to HIV, NASH, or Cancer the stock price as a percentage of value predicted a 20% probability of winning an EUA. The hesitancy of the FDA to move forward without more data probably makes sense, because not one company in the world has gotten an EUA for mild to moderate because it is NOT an unmet medical need. Furthermore, investors should really dig deep into the FDA’s thinking because they no longer seem concerned if it works, but who would be best suited to use it if diagnosed. They have to be well aware that if they approved it the mild to moderate population could suck up so much supply that the severe that really need the drug could be left out in the cold.
Severe Critical trial results are due to be released in the coming 3 to 4 weeks. So with the anticipated stock decline today investors are pricing in an even lower probability of approval in the CD12 trial. Now with the parade of doctors on the call each staking their lifelong reputations that the drug works perhaps investors will ask if this level of pessimism is really warranted. In the coming weeks, the stock could appreciate noticeably toward a binary readout in October. To the doctors and even CEO this drug will get approval this year, but the investing public has essentially thrown a temper tantrum. Investors are overlooking fundamental truths.
- The drug works.
- NASDAQ uplisting in process
- MHRA is positive on funding trials for EAMS patients in the U.K.
- NASH trial to start soon
- Long Hauler Study to start soon
- At least 4 journal articles submitted for publication
- Leronlimab crosses the blood-brain barrier
- GILD bought a comparable drug platform with an inferior safety profile for $21 billion
Investor’s who are interested in the fastest way to approve should get over their Mild to Moderate tantrum. When it comes to FDA approval for COVID, does it really matter what level of treatment it is approved at initially? Speed and certainty comes first for CytoDyn and the FDA sees the clearest treatment indication for this drug’s effectiveness in more critical patients. The important message in this call that is overlooked is that there is progress and a clearer path to approval which should come about in a reasonable time period as outlined by the company. I for one appreciate the call and view the statements as positive as I think most people in the know do. It is unfortunate that patience is not a strong characteristic trait in investors. However, those that stand firm should be handsomely rewarded upon approval.
Related articles by this author:
CytoDyn Investors Sell Despite Positive Recommendation From DSMB And A More Certain Path To COVID-19 Approval
HIV Cure Challenger CytoDyn Takes On Gilead Sciences
CytoDyn’s Data: Approvable Drug Sets Stage For Near-Term Move Upward
Disclosure: I own shares in CYDY.




Its sad what was done with this stock. It goes to show how any given stock has unknown things and is unpredictable. That said, I am still hopeful and am waiting for traction and positive news.
What is sad is your ignorant pumping. Nothing was done to this stock, it went down as there was no science, no management, no clinical or regulatory path forward. And no, it does not go to show any stock has "unknown things and is unpredictable" - I've been bearish on this for over a year while you were shamelessly pumping.
I've lost too much money on $CYDY. I am thinking it may be time to dump, but am hoping it will recover more first. Overall, not happy. This was one of my worst buys this year.

I'm unsure whether the recent bump in the stock is a relief rally or up on cancer and other news of theirs. I think the bulls are still waiting for news to sink their teeth into.
Still pumping this garbage? Has failed every study, been scoled by the FDA for lying, has had 25 different firms file lawsuits, lost a distributor in US, and stock is now down to $1.40 or thereabouts. When you started pumping this stock, it was in the $4 range. Have you no shame?
$CYDY is not without risk (perhaps a bit too much). But there's no reward without some risk, and there is a lot of potential here. But it's also very concerning that CYDY has lost over half it's value. Why?

Thanks for the continued updates.
Here's an update.
www.cytodyn.com/.../cytodyn-receives-positive-dsmc-recommendation-after-interim
www.zerohedge.com/.../unrecognized-therapeutic-takes-dying-covid-19-table-30000-american-lives-hanging
Interim results were good. However they want the study finished. In reality this provides a clear path to approval with a positive finding half way through. It is sad so many are dying while we lollygag through approvals with a drug that has been proven to save some lives with an extremely high safety profile.
Some new news I found that you may find interesting as we wait for CYDY results. The article writer says he expects the official release of interim results on Tuesday next week. The other article says the WHO found that Remdesivir after an 11k study was ineffective at helping patients recover any faster and has no effect on mortality either. They put it in the same ineffective designation as hydroxychloroquine, the HIV combination of lopinavir and ritonavir, and interferon.
www.msn.com/.../ar-BB1a4MeV
insiderfinancial.com/.../180558/
Trump news is getting more people to look at Cytodyn today.
insiderfinancial.com/.../180495/
Trump is taking Remdesivir even though he's not on supplemental oxygen, REGN-COV2 an experimental antibody treatment from Regeneron, zinc, vitamin D, famotidine (acid reflux), melatonin (for sleep), and aspirin so far.
www.msn.com/.../ar-BB19EVNe?ocid=msedgntp
But what does this drug have to do with Regeneron's drug?
He took REGN-COV2 as well and supposedly invested in the company too.
See the article the author linked to. But the author, @[Moon Kil Woong](user:5208), should write about it here as well.
Here is a short on the controversy about Trump using premature babies to cure himself. He did invest in Regeneron. They do use premature baby tissue. This is an article that is a defense regarding Regeneron's drug.
They claim their product came from premature baby organ cells so it is not a stem cell. Furthermore, they claim their drug isn't a cell but was derived from premature baby organ cells so it's not a premature baby, They just grew or took it from a premature baby organ cell they manipulated and that doesn't count. It's your call if parts of a cell duplicated from a premature baby's organ is still using a part of a premature baby for a drug. this is why it takes time to manufacture it in quantity. Trump has been cited promoting this and calling it a "miracle".
heavy.com/...
Sadly, if Cytodyn is approved it will show that there are solutions that we don't need to use aborted fetus experiment parts in a Corona cure.
Here is the links if you want to read more about this. heavy.com/.../regeneron-monoclonal-antibodies-not-from-human-fetal-embryo-stem-cells/ www.thecut.com/.../...oped-using-fetal-tissue.html
Trump was on supplemental oxygen, the white house has since confirmed.
Thanks you are correct although getting the truth is tough since they are forcing doctors to sign NDAs and Trump won't disclose details including information on exactly when he got infected.
Why?
Scientific knowledge that Cytodyn pumpers dont know or deliberately obfuscate - neutrophils in COVID19 are determined by CCR8, and not CCR5. See article below. Leronlimab binds CCR5, not CCR8. Which is why other than Cytodyn, NOBODY IN THE SCIENTIFIC OR PHARMA COMMUNITY trusts cytodyn data.
www.frontiersin.org/.../full
One article doesn't make scientific fact. This is why there is a scientific procedure, not conjecture being studied. Your arguments saying things aren't worth studying because you found something that fits your conclusion is not worth studying because you found something that fits your conclusion is not scientific and undermines what the FDA is doing, authorizing and managing clinical studies to determine scientific fact.
And no, Cytodyn isn't the only one identifying this link to fight Covid. It so happens to be the first drug going through the FDA approval to help those with Covid.
https://pubmed.ncbi.nlm.nih.gov/32511656/
www.wired.com/.../why-does-covid-19-make-some-people-so-sick-ask-their-dna/
www.peakprosperity.com/.../any-populations-with-natural-genetic-resistance-to-covid-19/
magazine.jhsph.edu/.../clues-covid-19-severity-may-lie-our-genes
www.pharmacytimes.com/.../novel-drug-may-reduce-inflammation-in-critical-covid-19-patients
What a poor response. You DID NOT SHOW A SINGLE PEER REVIEWED INDEPENDENT ARTICLE ON CCR5 AND COVID19. We are not talking about HIV but COVID19. Stay on topic, will you, and stop dissembling the truth?
Your article is making the case that neutrophils determine the severity of COVID-19. Severity is clearly correlated to the comorbidities of COVID-19. To prove that point there has to be some linkage between the comorbidities and neutrophils. When they do the UMAP presentation they use a healthy person. If the goal of the article is talking about predicting severity is should have compared to a diabetes patient or a hypertension patient. It did not. The comparison to covid state in the article is just an observation of what the end result of the disease is. If we follow your logic then blocking neutrophils will cure the disease. That is not the case. It will would help but its not a panacea. Neutrophils are just one of many immune cells. Blocking CCR5 results in blocking the macrophages that are primarily responsible for the signaling cascade. It is a multi pronged approach. That’s what leronlimab does. Regarding peer review, I will take wired and others journalistic integrity over an article that in no way says CCR5 is no good and which you wrongly assume blocking neutrophils will cure the disease which it won't. You are free to look on google regarding CCR5 and Covid. There are a lot and papers that are downloadable. However, I don't link them because people don't wantto go to a ling that downloads a scholarly paper to read. If you want you can go to research square and look up: Disruption of the CCL5/RANTES-CCR5 Pathway Restores Immune Homeostasis and Reduces Plasma Viral Load in Critical COVID-19 to read a professional scolarly rticle if you think the tons of press articles on this are garbage along with the tons of doctors cited in them. https://insight.jci.org/articles/view/139834 www.ncbi.nlm.nih.gov/pmc/articles/PMC7277012/ www.ncbi.nlm.nih.gov/pmc/articles/PMC7406242/ air.unimi.it/.../...EAE6347C985.suir-unimi-prod-02 I will not due your homework anymore but posted what I did as a service to all those who want to know the truth about your incorrect allegation against the scientific nature of Covid. Don't bother the informed with outlandish accusations.
What an ignorant and biased rant. Look at these reviews on the status of immune response to COVID19. These are by independent scientists who, unlike you or Cytodyn, have no conflict of interest. They are peer reviewed and are in world class journals Sciene, Lancet. CCR5 is not a pathway that anyone of repute espouses.
www.sciencedirect.com/.../S1074761320301837
science.sciencemag.org/content/369/6508/eabc8511
www.thelancet.com/.../fulltext#seccestitle60
Here is yet another article for the readers if they want to know more about CCR5 since Desai continues to ignore data on the topic. www.medrxiv.org/.../2020.05.02.20084673v1.full.pdf
Written by Cytodyn insiders!! What conflict of interest!! Zero credibilty!! LOL!!!!
Not everyone against CYDY is a short. Some like me dislike lies and respond to restore clarity and science.
1. The drug works. In HIV, yes. In COVID19, no. It MISSED THE PRIMARY ENDPOINT
2. NASDAQ uplisting in process. Unlikely. IS NOT MEETING CRITERIA.
3. MHRA is positive on funding trials for EAMS patients in the U.K. MORE GOEBBLES DOUBLESPEAK.
4. NASH trial to start soon. That is a problem as the company is running out of money, and has no expertise in NASH. Most importantly, SCIENCE DOES NOT SUPPORT NASH.
5. Long Hauler Study to start soon. SEE 1 ABOVE. WON'T WORK.
6 At least 4 journal articles submitted for publication. YET NOTHING PUBLISHED.
7. Leronlimab crosses the blood-brain barrier. SO WHAT? NO SCIENCE TO SUPPORT ANY CNS INDICATION.
8. GILD bought a comparable drug platform with an inferior safety profile for $21 billion. BUT IMMUNOMEDICS DRUG WORKS, LMAB IS NOT AS GOOD. IF LMAB IS SO GOOD, WHY HAS NO PHARMA COMPANY APPROACHED IT? NO VC?
9. MANAGEMENT HAS NO DRUG DEVELOPMENT EXPERIENCE. ONLY EXPERIENCE IN BEATING WIVES AND SELLING FAKE INDIAN ARTIFACTS.
Yawn.
1. Look at phase II results. It exhibited traits that existing drugs don't address and met the safety profile it was meant to prove. Even so, with a small sample size as a phase II it almost met the primary endpoint which is not expected. Read FDA results. I agree that it may be suitable for HIV.
2-3. Nasdaq approval isn't off the table and the major concern is price volatility which may be moderated especially after an approval as will better funding options just like all pharmaceutical companies. That said, it has plenty of cash to operate currently and will make adjustments as they see fit.
4-5. NASH trials are suppose to determine NASH results as are other study results. The FDA has not cited any of their studies as deficient to prove their points.
6. It is funny you mention studies on this. There have been numerous pre-FDA study results by doctors which have been cited by bears as being indicative of its ability and not proof thus is why one does FDA studies which were used to get FDA studies authorized. Blaming the egg for not being a chicken is a rather dumb argument in my book. Here are some as you claim unpublished articles on the topic. You are welcome to google this subject yourself:
www.cytodyn.com/.../cytodyn-reports-strong-results-from-eind-covid-19-patients
www.targetedonc.com/.../leronlimab-shows-further-promise-for-covid-19-treatment-study-shows
apnews.com/.../5f340ac37ee044e098fd1a843cb46b08
http://www.bioquicknews.com/node/5315
www.biospace.com/article/clinical-catch-up-may-4-8/
7. This is just something that was disclosed recently and may be a sign it can help people who may suffer from Covid related brain hemorrhages. The science has yet to be studied in depth or proven one way or another. Thus its too early to call and is fine to announce.
8. Who are you to say one drug is better than another. This is what regulatory bodies are for. You are injecting opinion not science into the argument.
9. This is just spiteful bear ranting. It makes me wonder why I even bother responding to such comments. Write your own article and be judged on it by your peers.
Then don't respond. You must have a guilty conscience.
It is hilarious that you think responding to commenters on their own article is disingenuous. So you are saying not responding is showing what? That people can spout idiocracy without being corrected. If so, I'm sure you will say not responding is showing a guilty conscience too. If your logic is that anyone disagreeing with you is wrong it is more likely you are the one spouting falsehoods. I can equally say your mad posting on all boards spouting rumors and innuendo about Cytodyn shows both your bias and your position, not that it makes a difference. My readers aren't stupid.
It's very hard to know who/what to believe about $CYDY. There seems to be so much conflicting info.
Which is why you should do your own due diligence and not listen to pumpers.
I'd be interested in hearing more from other viewpoints. Any chance you will write an article on this topic @[Ketan Desai](user:4649)?
Agreed, I suggest people do a bit of their own research on this. The price is low partially due to the big dissonance between the bulls and the bears. Then follow the science.
Are you truly nothing more than a share holder and fan of this stock? Or has the company compensated you for these articles? It sounds like this CYDY has a lot of haters.
I am a shareholder. My compensation will be when the stock skyrockets. And yes, there are a lot of haters and the company is small so they don't know how to make their information clear to small investors. That's why I write such articles. To me, it seems pretty clear that the FDA is moving forward on CYDY for Covid as laid out by the disclosures and the company meetings. Unfortunately, others have different opinions and still others muddle the waters with misleading disclosures and speculation. This is what short interest does when it has nothing of substance. To me, in most cases, it is just a matter of time before they get approval. Thanks to the shorts those who get it at this price won't have to wait long to make a lot of money. I've been on this board and Seeking Alpha for a long time. You can look me up if you want. I mostly comment.
@[Chuckles 759](user:135315) and @[Chump Punk](user:135341), you'd probably enjoy this article.
Good article, but my enthusiasm for $CYDY has really waned after reading some of @[Ketan Desai](user:4649)'s comments.
Loading comments, please wait...