Blockbuster SAR-CoV2 Disrupters Pre-ICU / Admitted ICU Patient Treatments & COVID-19 T-Cell Vaccine Horizon
Blockbuster disrupters in the fight against COVID-19
Cell therapies against COVID-19
Globally, the healthcare industry is using every weapon in its armory to suppress the threat from the novel SAR-CoV2 virus, including the use of living therapies such as natural killer (NK) cells, T-cells, stem cells, and exosomes as developed out of Capricor Therapeutics, Inc., (CAPR). While many novel approaches are being investigated, stem cells - mesenchymal stem cells (MSCs) in particular - are showing intriguing potential for the treatment of COVID-19.
Disrupters for those battling COVID-19 and complications from having a positive Coronavirus test
MSCs are receiving attention because past studies and the FDA emergency approval of CAP-1002 Cedars-Sinai Medical Center as ongoing study have found the secretions from MSCs to be effective at treating inflammation and cytokine storms. From early-stage studies, it appears that MSC may exert beneficial effects, potentially by improving the lung microenvironment, inhibiting immune system over-activation, promoting tissue repair, protecting lung alveoli epithelial cells, preventing pulmonary fibrosis, or improving lung function.
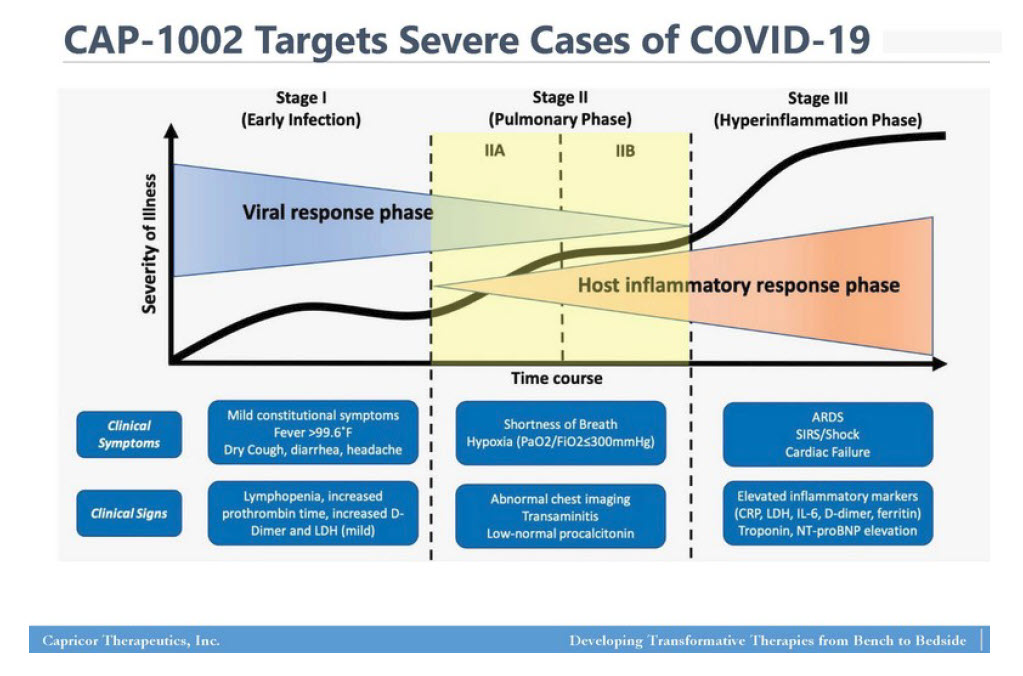
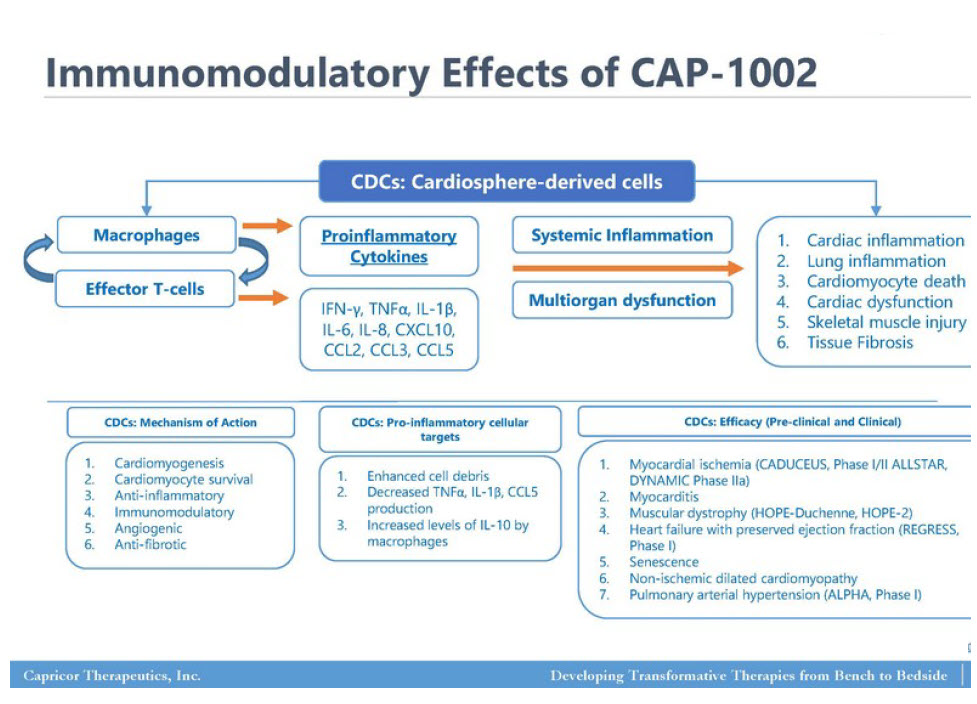
As leading examples, with Capricor Therapeutics, Inc., CAPR DMD CAP-1002 having synergistic and potentiated effects with the current COVID-19 Treatment Protocols, Dexamethasone/Steroid/Antimicrobial therapies as observed on severally ill ICU Intubated patients. These ICU SAR-CoV2 patients having block-buster reported by Cedars-Sinai Medical Center treating physicians in CAP-1002 relieving Pulmonary Hypertension, with a significant effect on supporting COVID-19 damages to ICU admitted patients having severe B/P low pressures affecting cardiac functions, but with CAP-1002, the cardiac injection fractions; had stabilized returning a more normal cardiac function, taking noted assistive reduction on cytokine storm effects to the Kidneys and Liver functions.
Furthermore, Athersys, Inc. (ATHX) has also reported positive outcomes in other studies using MSCs for treating respiratory, cardiac, kidney, and liver disease, with high expectations with Alhersys, Inc. Mesoblast's Remestemcel-L has proved to be effective in treating advanced respiratory distress. Athersys and Mesoblast are enrolling for 300 and 400 patient trials, respectively - two of the largest stem cell trials to date against COVID-19. In total, at least 26 companies are exploring cell therapy products against COVID-19 and its complications.
In recent months, there has been a flurry of activity within the clinical trial sector. When all trial types are considered, there are over 400 studies exploring approaches to diagnosing, treating, or preventing COVID-19. ClinicalTrials.gov has also reported at least 15 trials leveraging stem cells against COVID-19.
(Click on image to enlarge)
Provided by Capricor Therapeutics, Inc. (c) 2020
Why you need to know Dr. Stephen J. Gould from John Hopkins, moving forward exosome research & technology
COVID-19 is the largest pandemic in modern history, with more than ten million confirmed cases and a half million deaths in under six months.
While there is not yet an approved treatment for COVID-19, the scientific, medical, and regulatory agencies are making heroic efforts to bring out new medicines. Numerous COVID-19 product categories have emerged, including vaccines, antibodies, antivirals, repurposed drugs, RNA-based drugs, and cell-based therapies, as well as other approaches, such as enzymes, peptides, and glycoproteins.
This report explores each of these product development categories in detail, presenting the products under development and timelines for them to come to market. Special attention is given to the critical role of cell-based therapies in the management of the global pandemic.
Keeping focused on why M&A with PT $24 - $32 Dollars a share upside
(Click on image to enlarge)
(Click on image to enlarge)

COVID-19 Treatments & Technologies
To accelerate the development of therapies against COVID-19, the repurposing of existing drugs is being explored by numerous market competitors. For example, Barcitinib is being explored because of its anti-inflammatory effect and possible ability to reduce viral entry. A specific dose of the anti-HIV combination, Lopinavir-Nitonavir, is now in clinical trials with Arbidol or Ribavirin. Remdesivir, developed by Gilead Sciences (GILD), was earlier tested in patients with Ebola virus and has shown promise in animal models for MERS and SARS. Remdesivir has reached Phase III in the U.S. and China.
Favipiravir, a purine nucleoside leading to inaccurate viral RNA synthesis, was previously developed by Toyama Chemical of Japan and has now been approved for a clinical trial as a drug for the treatment of COVID-19. Chloroquine has shown itself to be effective in treating COVID-19 in China.
Dozens of companies are rushing vaccine development and proceeding toward clinical trials. As select examples, the U.S. NIH initiated a Phase I trial in Seattle evaluating an investigational vaccine (mRNA-1273) created by NIAID scientists and their collaborators at Moderna, Inc (MRNA). Sanofi (SNY) and Regeneron (REGN) launched a Phase II/II trial in New York evaluating the IL-6 targeted Kevzara. Inovio Pharmaceuticals (INO) is advancing its vaccine into human trials within the U.S. and intends to produce one million doses of it by the end of the year.
Summary
Cell Therapies Against COVID-19
Globally, the healthcare industry is using every weapon in its armory to suppress the threat from the virus, including the use of living therapies such as natural killer (NK) cells, T-cells, stem cells, and exosomes. While many novel approaches are being investigated, stem cells - mesenchymal stem cells (MSCs) in particular - are showing intriguing potential for the treatment of COVID-19.
MSCs are receiving attention because past studies have found the secretions from MSCs to be effective at treating inflammation and cytokine storms. From early-stage studies, it appears that MSC may exert beneficial effects, potentially by improving the lung microenvironment, inhibiting immune system over-activation, promoting tissue repair, protecting lung alveoli epithelial cells, preventing pulmonary fibrosis, or improving lung function.
As leading examples, Athersys, Inc. has reported positive outcomes in other studies using MSCs for treating respiratory disease, and Mesoblast's Remestemcel-L has proved to be effective in treating advanced respiratory distress. Athersys and Mesoblast are enrolling for 300 and 400 patient trials, respectively - two of the largest stem cell trials to date against COVID-19. In total, at least 26 companies are exploring cell therapy products against COVID-19 and its complications.
In recent months, there has been a flurry of activity within the clinical trial sector. When all trial types are considered, there are over 400 studies exploring approaches to diagnosing, treating, or preventing COVID-19. ClinicalTrials.gov has also reported at least 15 trials leveraging stem cells against COVID-19.
We will give our current hit-list in our next article dealing with biopharmaceuticals as great M&A candidates well past the vaccine and med/cocktail for COVID-19 has been put behind us.
Disclosure: None.




Lots of good info here, thanks.
Thanks for the complement!
What will bring all back to work! Joe Biden is clueless... Keeping the eyes on the prize! POTUS Capricor, CAPR yesterday, beat on all numbers and has brought a VLP Vaccine into the long-term solutions FDA TRIALS CAP-1002. talkmarkets.com/.../blockbuster-sar-cov2-disrupters-pre-icu--admitted-icu-patient-treatments--covid-19-t-cell-vaccine-horizon PT $14 - $24 3-12 month outlook.
Good connecting article for why the PT had hit the 52 weeks high $12.32 and maybe the reason for the August 6th or 8th earnings report in jacking the share price towards becoming a true M&A candidate or given the blessings of the FDA's approval for CAP-1002 BLA filing for uses.
READ:
endpts.com/microcap-capricor-soars-on-interim-phii-dmd-data-showing-functional-benefit-for-older-patients/
Very Important *****FYI for those seeking the best and earliest buy for both MESO with their BLA pending FDA approval on Remestence-L and CAPR CAP-1002 are just a few we are closely watching.
READ:
bioinformant.com/capricor-developing-treatment-dmd/ Inivio INO just got their BLA approval for their HPV suite of critical advancements in helping those stricken with Throat complications of HPV and that in critically advancing their VGX-3100 towards getting FDA final approvals.
Capricor CAPR has a few irons in the fire coming up when they present their upcoming second-quarter earnings and corporation pipeline status.
READ:
talkmarkets.com/.../blockbuster-sar-cov2-disrupters-pre-icu--admitted-icu-patient-treatments--covid-19-t-cell-vaccine-horizon
The first big iron for CAPR comes with a pending BLA for getting or meeting Orphan Drug Approval Status for their CAP-1002 for male boys and younger adult males with Duchenne muscular dystrophy (DMD) in 7 Major Markets.
This Iron is also quite remarkable with the incredible successes from the uses of MSCs and Exosome biotechnology advancements and the repurposing of their most current pipeline candidates awaiting approvals from the FDA for the accepted treatment protocols COVID-19 SARSCoV2; the eventual Orphan Drug Status for both Capricor CAPR CAP-1002 and Mesoblast LTD MESO for their Remestence-L.
Again, both MESO and CAPR for battling and achieving a true Disrupter Status in this war against China's born novel Coronavirus affecting our entire plant, since possibly October of 2019, when China kept this secret biological weapon hidden from the rest of the entire living masses around our world economies.
Keep up with the currently approved study for CAPR below as a link is provided. ClinicalTrials.gov identifier (NCT number): NCT04338347
READ:
https://clinicaltrials.gov/ct2w/NCT04338347
Very Important *****FYI for those seeking the best and earliest buy for both MESO with their BLA pending FDA approval on Remestence-L and CAPR CAP-1002 are just a few we are closely watching. READ: bioinformant.com/capricor-developing-treatment-dmd/ Inivio INO just got their BLA approval for their HPV suite of critical advancements in helping those stricken with Throat complications of HPV and that in critically advancing their VGX-3100 towards getting FDA final approvals. Capricor CAPR has a few irons in the fire coming up when they present their upcoming second-quarter earnings and corporation pipeline status. READ: talkmarkets.com/.../blockbuster-sar-cov2-disrupters-pre-icu--admitted-icu-patient-treatments--covid-19-t-cell-vaccine-horizon The first big iron for CAPR comes with a pending BLA for getting or meeting Orphan Drug Approval Status for their CAP-1002 for male boys and younger adult males with Duchenne muscular dystrophy (DMD) in 7 Major Markets. This Iron is also quite remarkable with the incredible successes from the uses of MSCs and Exosome biotechnology advancements and the repurposing of their most current pipeline candidates awaiting approvals from the FDA for the accepted treatment protocols COVID-19 SARSCoV2; the eventual Orphan Drug Status for both Capricor CAPR CAP-1002 and Mesoblast LTD MESO for their Remestence-L. Again, both MESO and CAPR for battling and achieving a true Disrupter Status in this war against China's born novel Coronavirus affecting our entire plant, since possibly October of 2019, when China kept this secret biological weapon hidden from the rest of the entire living masses around our world economies. Keep up with the currently approved study for CAPR below as a link is provided. ClinicalTrials.gov identifier (NCT number): NCT04338347 READ: https://clinicaltrials.gov/ct2/show/NCT04338347
Good article, but that headline is a mouthful. I think you would have gotten far more clicks with something more easily digestible/catchy.
I agree, the title of the article was a mouthful! I had a hard time shorting it, mostly due to the sure nature of covering a myriad of treatments and vaccines for ongoing war.
I will use my reply this morning, in taking efforts on getting out my frustrations for a NOVEL CORONAVIRUS that has killed hundreds of thousands, and yet has not met it's match to date.
Yes, I said WAR--being waged upon China's Deadly Gift--sent around the world with a Kiss...
China's well documented "Evil Intents" by the very nature of evil, as driven China determination in becoming the one and only superpower over our free democracies we all seemingly hold so dear.
I say this, because, it seems our LEFT-WING MEDIA and the Tech-Valley of Google's, Amazon's, Apple's, Twitter's, and a host of Democratic DNC elected Governor's, Mayor's, and elected officials throughout the many states and cities; seeing out and out rioting, causing untold damages throughout our United States.
Just getting out my frustrations this morning. I just can't believe the lengths the LEFT-WING Media has taken in trying to effect a presidential election in 2020.
We can all agree, when one can stand behind the cause of when an African American, who had been suffocated with a lethally used choke hold--as the one loan officer wouldn't release pressure--as many were yelling at the other two law enforcement officers to stop George's death as he was killed in Minneapolis, Minnesota, George Floyd's death, at the hands then, three identified law enforcement officers, with one actually being the stone blooded killer.
NO ONE can disagree with the outrage in the senseless killing of George Floyd, in wear legal protest is quite a correct response.
We in NO WAY or in ANY SHAPE, CONDONE what has gone on behind those that lead BLM condoning the extremes of lawlessness, an increase in civilian death's along with those wearing the BLUE as the violence seen over these past several months And within their past history's.
NO ONE should stand-by and allow lawlessness and outright Anarchies, as being played out in Portland, Seattle, Chicago, and other televised news coming out from a trusted NEWS SOURCE of FOX News; as I recommend many to turn towards, if they want the truth and not the FAKE NEWS Agendas.
Yes, FAKE NEWS AGENDAS being waged against our own 1st amendment rights, and though, I do find some other news outlets not afraid in getting out the truth behind all these very Evil and Diabolical efforts being played out right before all of our very eyes.
The coronavirus disease of the 2019 pandemic caused by the SARS-CoV-2 virus continues to inflict significant morbidity and mortality around the globe. A variety of cardiovascular presentations of SARS-CoV-2 infection have been described so far. However, the impact of SARS-CoV-2 on the right ventricle is largely unknown. Due to its pathophysiologic relevance, the right ventricle finds itself in the eye of the storm of coronavirus disease of 2019, placing it at a higher risk of failure. Increased afterload from acute respiratory distress syndrome and pulmonary embolism, negative inotropic effects of cytokines, and direct angiotensin-converting enzyme 2-mediated cardiac injury from SARS-CoV-2 are potential mechanisms of right ventricle dysfunction in coronavirus disease of 2019. Early detection and treatment of right ventricle dysfunction may lead to decreased mortality and improved patient outcomes in coronavirus disease of 2019. MOBIL COVID-19 PROTOCOLS: https://mobile.health.mil/asp/#/covid-19
"Significant" is subjective. Put this into perspective! Only 9/1,000 of 1 percent of the world population "may" have died from C-19. We will never know the true number because we cannot trust the numbers reported by politicians, or bureaucratic scientists and doctors. Death is inevitable and when you consider that, according to the most "trustworthy" government agency, the CDC, 80% of those who "may" have died from C-19 were over 65 years old and the UN, another "trustworthy" entity, which says that those over 80 years old are 5 times more likely to die, it's not as "significant" as you may think. On the other hand, the flu, for which a vaccine exists, kills people of all ages, even health young people. Again, put things into perspective so you can see the bigger picture.
Shh, don't tell @[DRM](user:130312), he doesn't really believe in the morbidity of COVID-19. But I'm glad you are helping to spread the word.
God bless! Thank you : -)
The following link should be kept close at hand when dealing with COVID-19 concerns and timely SARS-CoV2 ICU Patient Treatment Protocols: www.ashp.org/.../ASHP-COVID-19-Evidence-Table.ashx Viewing the Thoracic Concerns: www.atsjournals.org/.../rccm.201908-1581ST
GOOD CONNECTIONS FOR STAYING ON-TOP OF THIS WAR ON CHINA'S DEADLY GIFT SENT AROUND THE WORLD AS COVID-19: www.covid19-druginteractions.org/prescribing-resources liverpool-covid19.s3.eu-west-2.amazonaws.com/lnilz6wi5y5qxy4qw9l5zek8tzvc
Good Review for Capricor CAP-1002 Study as further will be released within this next reporting qtr. link.springer.com/.../s00395-020-0795-1.pdf This drug has great efficacy for COVID-19 U.S. hot-spots or uptick of cases for a number of patients meeting ICU status of care. This SARS-CoV2 COVID-19 Infectious Diseases Society of America--Adobe.pdf for current standing protocol treatments has a phenomenal pulse of what doctors are facing on the frontlines as of the current date. www.idsociety.org/.../covid-19-guideline-treatment-and-management/ Trying to educate as many as I can so, the more you know, the more you can spread the word for those who are dealing with COVID-19 themselves, or have a family member or close friends diagnosed and admitted into the hospital. with SARS-CoV2 pre-ICU or already admitted into the ICU.
Loading comments, please wait...