AstraZeneca Says Its COVID-19 Vaccine Produces Robust Immune Response In Elderly Patients
Days after US regulators gave the AstraZeneca (AZN) - Oxford vaccine the green light to re-start its US-based trials, the FT is reporting new data that seems promising, even if it's still pretty preliminary.
The vaccine, which was considered the Western frontrunner until US authorities halted the trial after two participants in the UK trial came down with symptoms, has since ceded its lead in the US to Pfizer, though regulators in Europe are still working to expedite the approval process, virtually guaranteeing that the most high-risk groups in the EU (health-care workers, the elderly, and the ill) should start receiving it by the end of the year, provided the final 'Phase 3' trial data confirm that the vaccine is, in fact, effective at preventing disease without compromising safety (at least, in the short term).
But on Monday morning, the FT reported that the vaccine has produced an encouraging response in older patients, triggering production of antibodies and T-cells in older patients.
The discovery that the vaccine being developed by the University of Oxford, in collaboration with AstraZeneca, triggers protective antibodies and T-cells in older age groups has encouraged researchers as they seek evidence that it will spare those in later life from serious illness or death from the virus.
Age has emerged as the principal risk factor for a severe bout of Covid-19. However, the immune system weakens with age, raising concerns that the very group that most needs the protection of a vaccine may generate the least effective response to one.
People aware of the results from so-called immunogenicity blood tests carried out on a subset of older participants say the findings echo data released in July that showed the vaccine generated “robust immune responses” in a group of healthy adults aged between 18 and 55. The earlier findings showed that the vaccine induced two forms of human immune response — generating antibodies and T-cells — for at least 56 days, according to an analysis published in The Lancet.
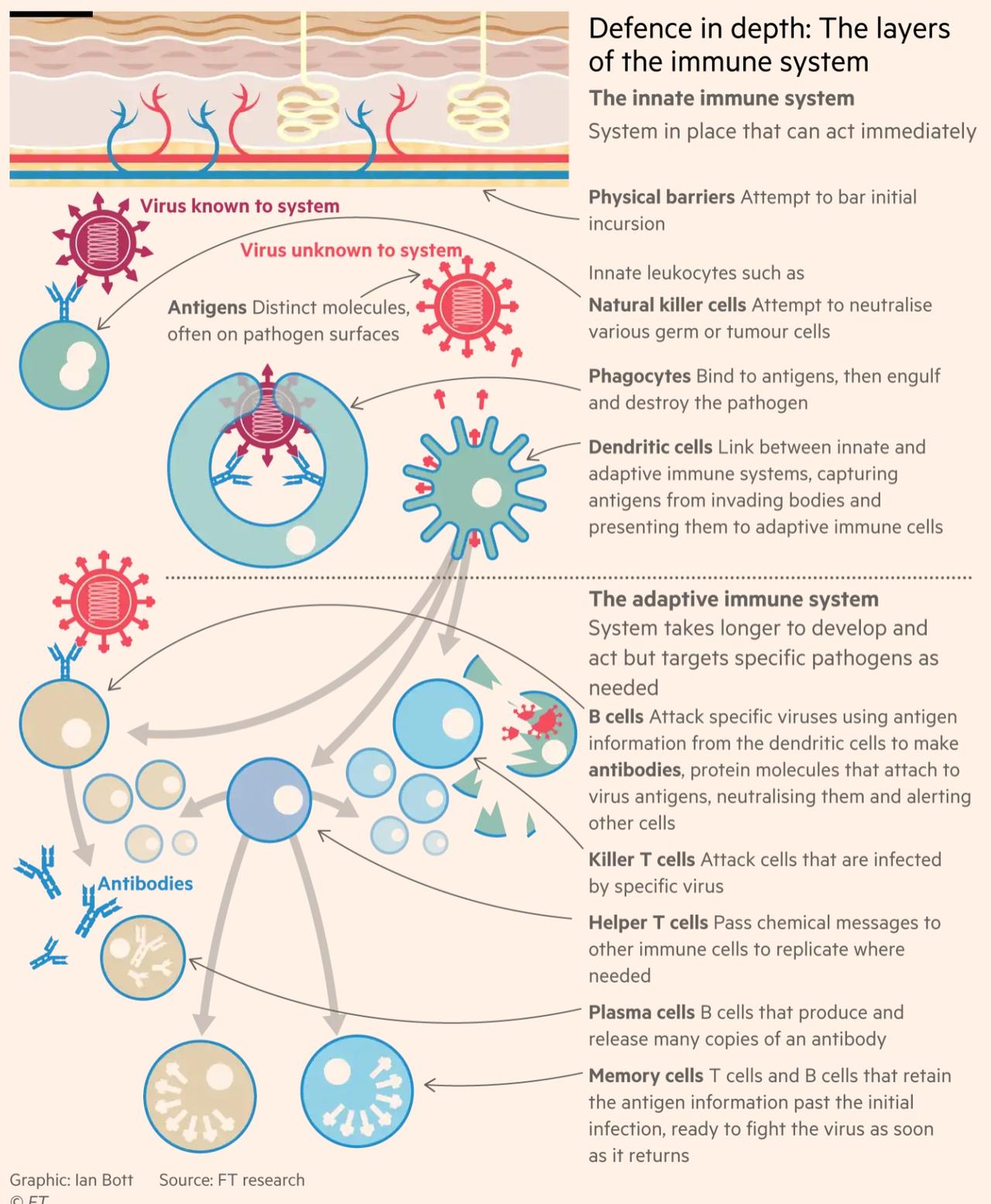
In an infographic, the FT illustrates how the immune system responds to the virus.
(Click on image to enlarge)
While at least one source corroborated the company's optimistic spin on the data, another scientist quoted by the FT cautioned that the data are hardly conclusive. And for now, at least, it's impossible to make a statement about timing with certainty.
The "Phase 3" trial data isn't even in yet, and besides, nobody can say for sure how British regulators might interpret the data once it is.
But investors and the public will be watching, probably with a great deal of intensity considering that President Trump's comments about how little we know w/r/t viral immunity during the final presidential debate with Joe Biden have brought that issue back to prominence.
This hasn't stopped the British health system from placing an extremely high degree of confidence in the vaccine. Staff at a major London hospital trust have been told to be ready to receive the first batches of the vaccine being developed by the University of Oxford and AstraZeneca, according to British tabloid the Sun.
Disclaimer: Copyright ©2009-2020 ZeroHedge.com/ABC Media, LTD; All Rights Reserved. Zero Hedge is intended for Mature Audiences. Familiarize yourself with our legal and use policies every ...
more