Sensex Opens Marginally Up; Wipro Gains Over 3.3%
Asian equities are trading mixed today as investors awaited the release of China's second-quarter GDP. Hong Kong's Hang Seng surged over 95 points. China's Shanghai Composite which is trading at 3,182 has slipped 40 points. The US markets ended the week on a high note. The Dow and S&P 500 hit record highs on Friday after weak economic data dulled prospects of more interest rate hikes this year.
Back home, share markets in India have opened the day on a positive note. The BSE Sensex is trading higher by 62 points while the NSE Nifty is trading higher by 19 points. The BSE Mid Cap Index and BSE Small Cap index opened the day up by 0.4% & 0.3% respectively.
Barring FMCG stocks, all sectoral indices have opened the day in the green with energy stocks and capital goods stocks leading the pack of gainers. The rupee is trading at 64.45 to the US$.
Wipro share price surged over 3.3% on the reports that its board will consider a proposal for buyback of equity shares on 20 July.
With this, Wipro joins the growing roaster of Indian IT firms that have announced buyback offers to return surplus cash on their books to their shareholders.
Pharma stocks opened the day on a mixed note with Cadila Healthcare and Dishman Pharma leading the losses. As per an article in a leading financial daily, Zydus Cadila has received a tentative nod from the US health regulator to market Fingolimod capsules used for treatment of multiple sclerosis.
The company has received tentative approval from the United States Food and Drug Administration (USFDA) to market Fingolimod capsules 0.5 mg.
The drug will be produced at the group's formulations manufacturing facility at pharma SEZ in Ahmedabad, the reports noted. As per market estimates, the US market for Fingolimod Capsules is approximately US$ 2.1 billion.
With this, the group now has over 120 approvals and has so far filed more than 300 Abbreviated New Drug Applications (ANDAs).
Meanwhile, Alkem Laboratories announced that the US health regulator has not issued any 'observation' post inspection of its Taloja facility in Maharashtra.
USFDA had conducted an inspection at the company's bioequivalence facility located at Taloja from 10 July to 14 July 2017.
Notably, USFDA alerts on Indian pharma companies have increased over the past few years. Regulators used to visit the plants every two years. Now they come every eight months.
Increasing inspections have led to a total of 41 import alerts in the past eight years. This clearly signifies increased USFDA scrutiny on Indian pharma firms. If that wasn't enough, increasing pricing pressure in the generics segment has dented realizations.
Expediting Drug Approval Process to be a Positive for Industry
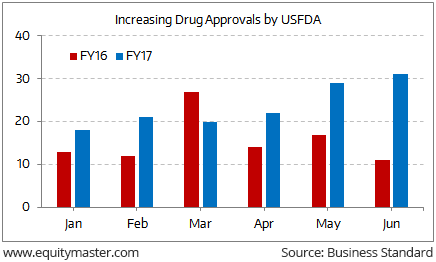
In this dull scenario, there appears to be some respite as the USFDA has expedited the drug approval process. Drug approvals for Indian companies have gone up 50% in the period from January to June 2017 compared to the same period last year.
Cadila Healthcare share price and Alkem Laboratories share price opened up by 1.4% & 1.8% respectively.
In another development, foreign investors have pumped in nearly Rs 110 billion in the capital markets in the first two weeks of this month on the back of the trouble-free rollout of GST and stimulating Indian economy.
The latest inflow comes following a net infusion of over Rs 1.62 trillion in the previous five months (February-June) on several factors.
Prior to that, such investors had pulled out over Rs 34.96 billion from debt markets in January.
According to the latest depository data, FPIs invested a net Rs 4.98 billion in equities during July 3-14, while they poured Rs 104.05 billion in the debt markets during the period under review, translating into a net inflow of Rs 109.03 billion (US$1.7 billion).
With the latest inflow, the total investment in the capital markets (equity and debt) has reached Rs 1.6 trillion (over US$24 billion) this year.
Disclosure: None.



